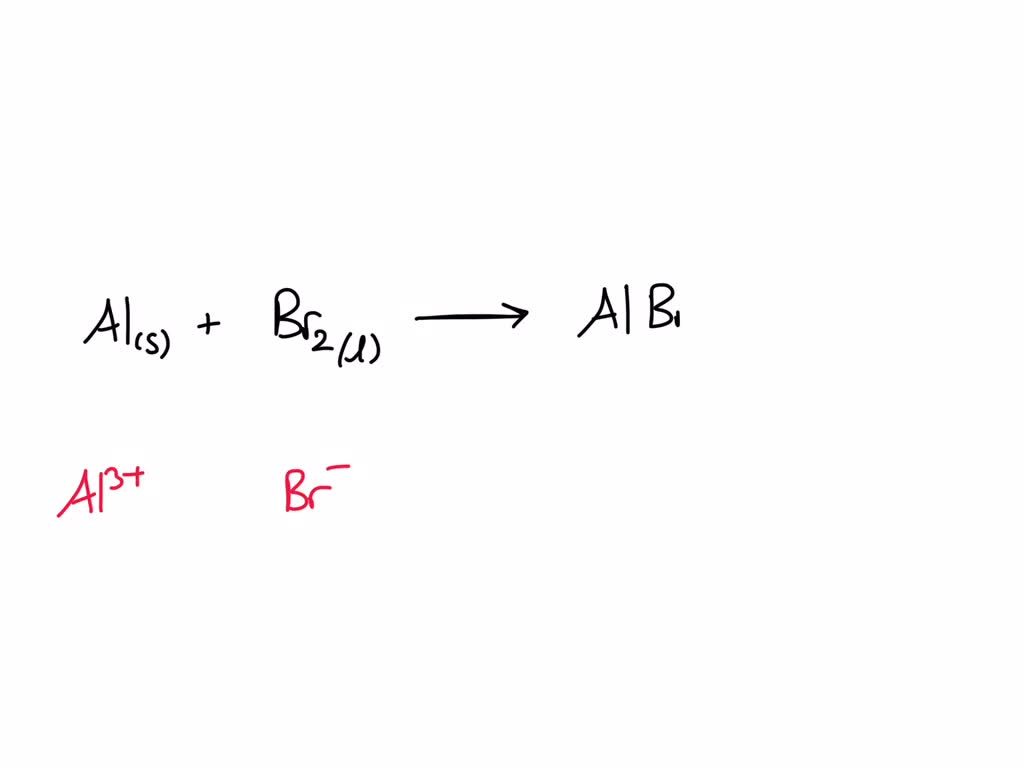Aluminium Chloride And Bromine Gas . Albr + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aluminium monobromide [albr] and three moles. Bromine gas = br2 b r 2. In order to sum to zero, you need to have three. Albr3 + cl2 → alcl3 + br2 a l b r 3 + c l 2 → a l c. Aluminum chloride = alcl3 a l c l 3. Accompanied by flames and coloured ‘smoke’,. Dium phosphate and calcium chloride react to form. Now let's write the chemical equation: Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. 3) sodium phosphate and calcium chloride react. Albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide [albr 3] and three moles of. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. 2) aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas.
from www.numerade.com
Dium phosphate and calcium chloride react to form. In order to sum to zero, you need to have three. Albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide [albr 3] and three moles of. 2) aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Accompanied by flames and coloured ‘smoke’,. Albr + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aluminium monobromide [albr] and three moles. Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Albr3 + cl2 → alcl3 + br2 a l b r 3 + c l 2 → a l c. Aluminum chloride = alcl3 a l c l 3. Bromine gas = br2 b r 2.
SOLVED Solid aluminum metal and diatomic bromine liquid react
Aluminium Chloride And Bromine Gas Albr + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aluminium monobromide [albr] and three moles. Accompanied by flames and coloured ‘smoke’,. 3) sodium phosphate and calcium chloride react. Albr + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aluminium monobromide [albr] and three moles. 2) aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Dium phosphate and calcium chloride react to form. Now let's write the chemical equation: Albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide [albr 3] and three moles of. Albr3 + cl2 → alcl3 + br2 a l b r 3 + c l 2 → a l c. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. Bromine gas = br2 b r 2. Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. In order to sum to zero, you need to have three. Aluminum chloride = alcl3 a l c l 3.
From www.youtube.com
Balancing Chemical Equations. Part 4 Aluminium bromide and chlorine Aluminium Chloride And Bromine Gas 3) sodium phosphate and calcium chloride react. Accompanied by flames and coloured ‘smoke’,. In order to sum to zero, you need to have three. 2) aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide [albr 3]. Aluminium Chloride And Bromine Gas.
From www.youtube.com
Reaction of Aluminium with Bromine YouTube Aluminium Chloride And Bromine Gas Aluminum chloride = alcl3 a l c l 3. Now let's write the chemical equation: Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. Albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide [albr. Aluminium Chloride And Bromine Gas.
From slideplayer.com
Chemical reactions particles & energy ppt video online download Aluminium Chloride And Bromine Gas In order to sum to zero, you need to have three. Albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide [albr 3] and three moles of. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and. Aluminium Chloride And Bromine Gas.
From www.youtube.com
Reaction of Chlorine with Aluminium YouTube Aluminium Chloride And Bromine Gas 3) sodium phosphate and calcium chloride react. Bromine gas = br2 b r 2. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. In order to sum to zero, you need to have three. 2) aluminum bromide and chlorine gas react to form aluminum chloride. Aluminium Chloride And Bromine Gas.
From www.youtube.com
How to Balance Al + Br2 = AlBr3 (Aluminum + Bromine gas) YouTube Aluminium Chloride And Bromine Gas In order to sum to zero, you need to have three. 2) aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Albr + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aluminium monobromide [albr] and three moles. Albr3 + cl2 → alcl3 + br2 a l b r 3. Aluminium Chloride And Bromine Gas.
From www.slideserve.com
PPT Balancing Chemical Reactions PowerPoint Presentation, free Aluminium Chloride And Bromine Gas Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. 3) sodium phosphate and calcium chloride react. Accompanied by flames and coloured ‘smoke’,. Albr3 + cl2 → alcl3 + br2 a l b r 3 + c l 2 → a l c. Albr + cl = alcl3 + br is a single displacement (substitution) reaction where. Aluminium Chloride And Bromine Gas.
From www.studypool.com
SOLUTION General chemistry 1 types of reaction Studypool Aluminium Chloride And Bromine Gas Dium phosphate and calcium chloride react to form. 2) aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. In order to sum to zero, you need to have three. Albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide [albr 3] and three moles of. Aluminum. Aluminium Chloride And Bromine Gas.
From fphoto.photoshelter.com
science chemical reaction redox aluminum bromine Fundamental Aluminium Chloride And Bromine Gas Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. 3) sodium phosphate and calcium chloride react. Albr3 + cl2 → alcl3 + br2 a l b r 3 + c l 2 → a l c. Accompanied by flames and coloured ‘smoke’,. Now let's write the chemical equation: 2) aluminum bromide and chlorine gas react to. Aluminium Chloride And Bromine Gas.
From www.numerade.com
SOLVED Solid aluminum metal and diatomic bromine liquid react Aluminium Chloride And Bromine Gas Aluminum chloride = alcl3 a l c l 3. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. Albr + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aluminium monobromide [albr] and three moles. Dium phosphate and calcium. Aluminium Chloride And Bromine Gas.
From www.slideserve.com
PPT Chemistry Unit 6 PowerPoint Presentation, free download ID6652385 Aluminium Chloride And Bromine Gas Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. 2) aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Accompanied by flames and coloured ‘smoke’,. 3) sodium phosphate and calcium chloride react. Albr + cl = alcl3 + br is a. Aluminium Chloride And Bromine Gas.
From fphoto.photoshelter.com
science chemical reaction redox aluminum bromine Fundamental Aluminium Chloride And Bromine Gas In order to sum to zero, you need to have three. Accompanied by flames and coloured ‘smoke’,. Now let's write the chemical equation: Bromine gas = br2 b r 2. 2) aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole. Aluminium Chloride And Bromine Gas.
From www.sciencephoto.com
Aluminium reacting with bromine Stock Image A500/0314 Science Aluminium Chloride And Bromine Gas Albr3 + cl2 → alcl3 + br2 a l b r 3 + c l 2 → a l c. Albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide [albr 3] and three moles of. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where. Aluminium Chloride And Bromine Gas.
From slideplayer.com
Chemical Reactions. ppt download Aluminium Chloride And Bromine Gas 3) sodium phosphate and calcium chloride react. Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Albr + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aluminium monobromide [albr] and three moles. Dium phosphate and calcium chloride react to form. In order to sum to zero, you need to. Aluminium Chloride And Bromine Gas.
From www.numerade.com
SOLVED Solid aluminum reacts with hydrochloric acid to produce Aluminium Chloride And Bromine Gas Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide [albr 3] and three moles of. 2) aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Accompanied by flames and coloured ‘smoke’,. Dium. Aluminium Chloride And Bromine Gas.
From ar.inspiredpencil.com
Bromine Liquid Formula Aluminium Chloride And Bromine Gas Albr3 + cl2 → alcl3 + br2 a l b r 3 + c l 2 → a l c. Albr + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aluminium monobromide [albr] and three moles. 3) sodium phosphate and calcium chloride react. Albr3 + cl = alcl3 + br2 is a single. Aluminium Chloride And Bromine Gas.
From brainly.com
Aluminum bromide and chlorine gas react to form aluminum chloride and Aluminium Chloride And Bromine Gas 3) sodium phosphate and calcium chloride react. Albr3 + cl2 → alcl3 + br2 a l b r 3 + c l 2 → a l c. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. Bromine gas = br2 b r 2. Now let's. Aluminium Chloride And Bromine Gas.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Aluminium Chloride And Bromine Gas 2) aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Accompanied by flames and coloured ‘smoke’,. Albr + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aluminium monobromide [albr] and three moles. Albr3 + cl2 → alcl3 + br2 a l b r 3 + c l 2 →. Aluminium Chloride And Bromine Gas.
From www.numerade.com
SOLVED Aluminum bromide and chlorine gas react to form aluminum Aluminium Chloride And Bromine Gas Bromine gas = br2 b r 2. Albr + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aluminium monobromide [albr] and three moles. Aluminum chloride = alcl3 a l c l 3. Dium phosphate and calcium chloride react to form. Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas.. Aluminium Chloride And Bromine Gas.
From www.masterorganicchemistry.com
Electrophilic Aromatic Substitutions Chlorination and Bromination Aluminium Chloride And Bromine Gas Accompanied by flames and coloured ‘smoke’,. 3) sodium phosphate and calcium chloride react. Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Dium phosphate and calcium chloride react to form. Albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide [albr 3] and three moles of.. Aluminium Chloride And Bromine Gas.
From material-properties.org
Aluminium and Chlorine Comparison Properties Material Properties Aluminium Chloride And Bromine Gas Accompanied by flames and coloured ‘smoke’,. In order to sum to zero, you need to have three. Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Bromine gas = br2 b r 2. Aluminum chloride = alcl3 a l c l 3. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where. Aluminium Chloride And Bromine Gas.
From www.adda247.com
Aluminium Chloride FormulaDefinition, Structure, Uses, Properties Aluminium Chloride And Bromine Gas Now let's write the chemical equation: Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide [albr 3] and three moles of. Albr3 + cl2 → alcl3 + br2 a l b r 3 + c l. Aluminium Chloride And Bromine Gas.
From www.youtube.com
Reaction of aluminum with bromine YouTube Aluminium Chloride And Bromine Gas 2) aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. Albr3 + cl = alcl3 + br is a. Aluminium Chloride And Bromine Gas.
From ar.inspiredpencil.com
Bromine Gas Equation Aluminium Chloride And Bromine Gas Now let's write the chemical equation: Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Aluminum chloride = alcl3 a l c l 3. Albr3 + cl2 → alcl3 + br2 a l b r 3 + c l 2 → a l c. Accompanied by flames and coloured ‘smoke’,. Bromine gas = br2 b r. Aluminium Chloride And Bromine Gas.
From slideplayer.com
Counting Naming Balancing Activity Series Reactions ppt download Aluminium Chloride And Bromine Gas Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide [albr 3] and three moles of. 2) aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Now let's write the chemical equation: Albr. Aluminium Chloride And Bromine Gas.
From www.numerade.com
SOLVED Write the correct skeleton chemical equation for the following Aluminium Chloride And Bromine Gas Albr + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aluminium monobromide [albr] and three moles. Bromine gas = br2 b r 2. Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Aluminum chloride = alcl3 a l c l 3. Albr3 + cl = alcl3 + br2 is. Aluminium Chloride And Bromine Gas.
From slideplayer.com
Balancing Word Equations ppt download Aluminium Chloride And Bromine Gas Albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide [albr 3] and three moles of. Dium phosphate and calcium chloride react to form. Albr3 + cl2 → alcl3 + br2 a l b r 3 + c l 2 → a l c. Aluminum bromide and chlorine gas react. Aluminium Chloride And Bromine Gas.
From askfilo.com
Problem 3 Aluminum reacts with chlorine gas to form aluminum chloride v.. Aluminium Chloride And Bromine Gas Now let's write the chemical equation: Albr + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aluminium monobromide [albr] and three moles. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. Aluminum bromide and chlorine gas react to. Aluminium Chloride And Bromine Gas.
From www.chegg.com
Solved Aluminum and bromine gas react according to the Aluminium Chloride And Bromine Gas Albr3 + cl2 → alcl3 + br2 a l b r 3 + c l 2 → a l c. In order to sum to zero, you need to have three. Albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide [albr 3] and three moles of. Accompanied by flames. Aluminium Chloride And Bromine Gas.
From www.slideserve.com
PPT CLASSIFYING CHEMICAL REACTIONS PowerPoint Presentation, free Aluminium Chloride And Bromine Gas Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Now let's write the chemical equation: 3) sodium phosphate and calcium chloride react. Albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide [albr 3] and three moles of. Bromine gas = br2 b r 2. Dium. Aluminium Chloride And Bromine Gas.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Aluminium Chloride And Bromine Gas 3) sodium phosphate and calcium chloride react. 2) aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Albr + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aluminium monobromide [albr] and three moles. Albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole. Aluminium Chloride And Bromine Gas.
From www.youtube.com
Reacting Bromine and Aluminum YouTube Aluminium Chloride And Bromine Gas Dium phosphate and calcium chloride react to form. In order to sum to zero, you need to have three. Albr + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aluminium monobromide [albr] and three moles. 2) aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Aluminum chloride = alcl3. Aluminium Chloride And Bromine Gas.
From hydrogengasgaosube.blogspot.com
Hydrogen Gas Hydrogen Gas With Bromine Gas Aluminium Chloride And Bromine Gas Now let's write the chemical equation: 3) sodium phosphate and calcium chloride react. Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Albr3 + cl2 → alcl3 + br2 a l b r 3 + c l 2 → a l c. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where. Aluminium Chloride And Bromine Gas.
From www.mozaweb.com
The reaction between aluminium and bromine Digital lesson Mozaik Aluminium Chloride And Bromine Gas Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Albr3 + cl2 → alcl3 + br2 a l b r 3 + c l 2 → a l c. In order to sum to zero, you need to have three. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles. Aluminium Chloride And Bromine Gas.
From slideplayer.com
Introduction to Chemical Reactions ppt download Aluminium Chloride And Bromine Gas Dium phosphate and calcium chloride react to form. Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. 2) aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. In order to sum to zero, you need to have three. Accompanied by flames and coloured ‘smoke’,. Albr3 + cl = alcl3 + br is. Aluminium Chloride And Bromine Gas.
From www.youtube.com
Bromine vs aluminum is insane! YouTube Aluminium Chloride And Bromine Gas In order to sum to zero, you need to have three. Albr3 + cl2 → alcl3 + br2 a l b r 3 + c l 2 → a l c. Albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide [albr 3] and three moles of. Dium phosphate and. Aluminium Chloride And Bromine Gas.