Calorie Lab Answers . When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. A calorie is the amount of heat (energy) required to increase the temperature of 1 gram of water by 1 ° c. Calorimeters can be used to find a substance’s specific heat. Calorimeters can be used to find a substance’s specific heat capacity. You will use the calorimetry lab gizmotm to determine the specific heat capacities of various substances. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. • read the lab thoroughly. This process is the basis of the technique.
from www.chegg.com
This process is the basis of the technique. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. A calorie is the amount of heat (energy) required to increase the temperature of 1 gram of water by 1 ° c. You will use the calorimetry lab gizmotm to determine the specific heat capacities of various substances. • read the lab thoroughly. Calorimeters can be used to find a substance’s specific heat capacity. Calorimeters can be used to find a substance’s specific heat. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up.
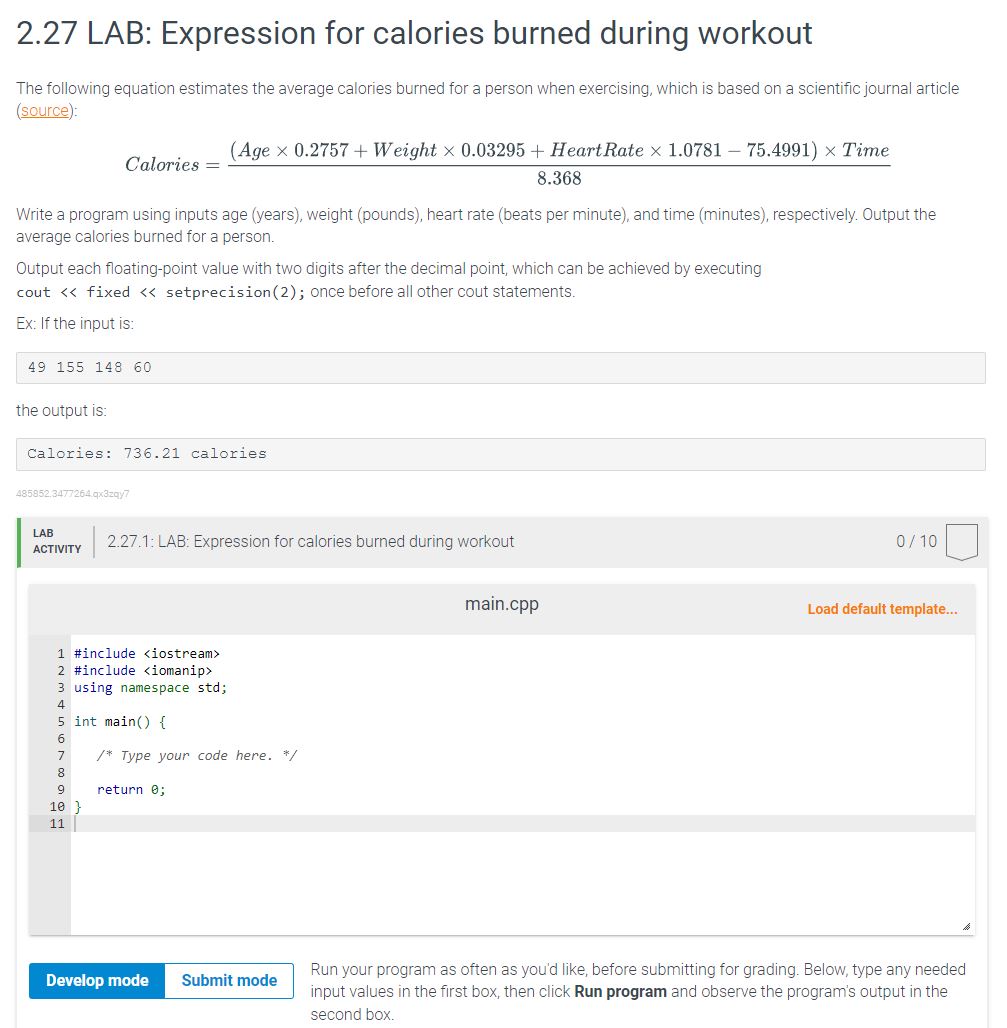
Solved 2.27 LAB Expression for calories burned during
Calorie Lab Answers You will use the calorimetry lab gizmotm to determine the specific heat capacities of various substances. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. You will use the calorimetry lab gizmotm to determine the specific heat capacities of various substances. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. A calorie is the amount of heat (energy) required to increase the temperature of 1 gram of water by 1 ° c. Calorimeters can be used to find a substance’s specific heat. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. • read the lab thoroughly. Calorimeters can be used to find a substance’s specific heat capacity. This process is the basis of the technique.
From www.slideshare.net
Calorimetry Lab Calorie Lab Answers • read the lab thoroughly. A calorie is the amount of heat (energy) required to increase the temperature of 1 gram of water by 1 ° c. This process is the basis of the technique. Calorimeters can be used to find a substance’s specific heat capacity. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing. Calorie Lab Answers.
From www.studocu.com
Experiment 25 Calorimetry Prelaboratory Assignment CHEM 005 Studocu Calorie Lab Answers You will use the calorimetry lab gizmotm to determine the specific heat capacities of various substances. Calorimeters can be used to find a substance’s specific heat. Calorimeters can be used to find a substance’s specific heat capacity. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. When a hot object is. Calorie Lab Answers.
From www.chegg.com
Solved Energy and Calorie Lab Energy and Calorie Lab Name Calorie Lab Answers 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. • read the lab thoroughly. Calorimeters can be used to find a substance’s specific heat capacity. You will use the calorimetry lab gizmotm to determine the specific heat capacities of various substances. When a hot object is placed in the calorimeter, heat. Calorie Lab Answers.
From www.transtutors.com
(Solved) Pre and post lab Estimating the Calorie Content of Nuts. Pre Calorie Lab Answers This process is the basis of the technique. Calorimeters can be used to find a substance’s specific heat capacity. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. You will use the calorimetry lab gizmotm to determine the specific heat capacities of various substances. A. Calorie Lab Answers.
From www.slideserve.com
PPT Food Energy Lab PowerPoint Presentation, free download ID5977359 Calorie Lab Answers 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. Calorimeters can be used to find a substance’s specific heat capacity. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. This process is the basis of the. Calorie Lab Answers.
From www.chegg.com
Solved 1.24 LAB Expression for calories burned during Calorie Lab Answers 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. This process is the basis of the technique. Calorimeters can be used to find a substance’s specific heat.. Calorie Lab Answers.
From www.formsbank.com
Lab Counting Calories printable pdf download Calorie Lab Answers Calorimeters can be used to find a substance’s specific heat. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. This process. Calorie Lab Answers.
From www.pinterest.com.au
Lab Activity Calculating Calories of Heat Lab activities, Free Calorie Lab Answers This process is the basis of the technique. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. When a hot object is placed in the calorimeter, heat. Calorie Lab Answers.
From www.chegg.com
Solved 2.15 LAB Expression For Calories Burned During Wo... Calorie Lab Answers You will use the calorimetry lab gizmotm to determine the specific heat capacities of various substances. Calorimeters can be used to find a substance’s specific heat capacity. A calorie is the amount of heat (energy) required to increase the temperature of 1 gram of water by 1 ° c. 1) if 0.315 moles of hexane (c6h14) is combusted in a. Calorie Lab Answers.
From www.scribd.com
Calorimetry Lab Explanation Chemistry Physical Sciences Free 30 Calorie Lab Answers You will use the calorimetry lab gizmotm to determine the specific heat capacities of various substances. This process is the basis of the technique. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. Calorimeters can be used to find a substance’s specific heat. When a. Calorie Lab Answers.
From www.studypool.com
SOLUTION Student exploration calorimetry lab vocabulary calorie Calorie Lab Answers When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. You will use the calorimetry lab gizmotm to determine the specific heat capacities of various substances. A calorie is the amount of heat (energy) required to increase the temperature of 1 gram of water by 1. Calorie Lab Answers.
From www.chegg.com
Solved Lab 5C CalorieResi Grade Section Instructor Calorie Lab Answers When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. This process is the basis of the technique. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. When a hot object is placed in the calorimeter, heat. Calorie Lab Answers.
From www.chegg.com
Solved Data Sheet Table 1 Calorie Testing PostLab Calorie Lab Answers When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. This process is the basis of the technique. A calorie is the amount of heat (energy) required to increase the temperature of 1 gram of water by 1 ° c. You will use the calorimetry lab. Calorie Lab Answers.
From www.chegg.com
Solved Expression for calories burned during workout 3.27 Calorie Lab Answers Calorimeters can be used to find a substance’s specific heat capacity. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. Calorimeters can be used to find a substance’s specific heat. You will use the calorimetry lab gizmotm to determine the specific heat capacities of various. Calorie Lab Answers.
From www.studypool.com
SOLUTION Student exploration calorimetry lab vocabulary calorie Calorie Lab Answers Calorimeters can be used to find a substance’s specific heat capacity. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. Calorimeters can be used to find a. Calorie Lab Answers.
From www.chegg.com
Solved 1.24 LAB Expression for calories burned during Calorie Lab Answers Calorimeters can be used to find a substance’s specific heat. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. A calorie is the amount of heat (energy) required to increase the temperature of 1 gram of water by 1 ° c. When a hot object. Calorie Lab Answers.
From sfsgyfbhpf.blogspot.com
Calorimetry Lab Gizmo Answers Activity C Calorimetrylabse Pdf Daniel Calorie Lab Answers When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. Calorimeters can be used to find a substance’s specific heat. This process is the basis of the technique. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the. Calorie Lab Answers.
From studylib.net
Calorimetry Lab Gizmo Calorie Lab Answers Calorimeters can be used to find a substance’s specific heat capacity. You will use the calorimetry lab gizmotm to determine the specific heat capacities of various substances. This process is the basis of the technique. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. A. Calorie Lab Answers.
From www.transtutors.com
(Solved) Pre and post lab Estimating the Calorie Content of Nuts. Pre Calorie Lab Answers When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. You will use the calorimetry lab gizmotm to determine the specific heat. Calorie Lab Answers.
From studylib.net
Calorimetry Worksheet Calorie Lab Answers This process is the basis of the technique. You will use the calorimetry lab gizmotm to determine the specific heat capacities of various substances. A calorie is the amount of heat (energy) required to increase the temperature of 1 gram of water by 1 ° c. • read the lab thoroughly. Calorimeters can be used to find a substance’s specific. Calorie Lab Answers.
From www.chegg.com
Solved 2.27 LAB Expression for calories burned during Calorie Lab Answers Calorimeters can be used to find a substance’s specific heat capacity. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. You. Calorie Lab Answers.
From www.studypool.com
SOLUTION Student exploration calorimetry lab vocabulary calorie Calorie Lab Answers You will use the calorimetry lab gizmotm to determine the specific heat capacities of various substances. • read the lab thoroughly. This process is the basis of the technique. A calorie is the amount of heat (energy) required to increase the temperature of 1 gram of water by 1 ° c. When a hot object is placed in the calorimeter,. Calorie Lab Answers.
From www.studocu.com
Calorie Lab write up Lab report following the inclass lab work Calorie Lab Answers A calorie is the amount of heat (energy) required to increase the temperature of 1 gram of water by 1 ° c. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. • read the lab thoroughly. When a hot object is placed in the calorimeter,. Calorie Lab Answers.
From www.studocu.com
Copy of Calorimetry Lab SE Name Karimah Edwards Date 18/11/ Student Calorie Lab Answers A calorie is the amount of heat (energy) required to increase the temperature of 1 gram of water by 1 ° c. Calorimeters can be used to find a substance’s specific heat. Calorimeters can be used to find a substance’s specific heat capacity. This process is the basis of the technique. When a hot object is placed in the calorimeter,. Calorie Lab Answers.
From www.youtube.com
calorie lab 2 3 2020 10 32 26 AM YouTube Calorie Lab Answers When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. You will use the calorimetry lab gizmotm to determine the specific heat capacities of various substances. Calorimeters can be used to find a substance’s specific heat capacity. 1) if 0.315 moles of hexane (c6h14) is combusted. Calorie Lab Answers.
From www.chegg.com
Solved Worksheet 12 Calculating Calories Directions Use Calorie Lab Answers 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. You will use the calorimetry lab gizmotm to determine the specific heat capacities of various substances. Calorimeters can be used to find a substance’s specific heat. When a hot object is placed in the calorimeter, heat energy is transferred from the object. Calorie Lab Answers.
From www.studypool.com
SOLUTION Gizmo student exploration calorimetry lab answer key Studypool Calorie Lab Answers A calorie is the amount of heat (energy) required to increase the temperature of 1 gram of water by 1 ° c. This process is the basis of the technique. Calorimeters can be used to find a substance’s specific heat capacity. • read the lab thoroughly. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing. Calorie Lab Answers.
From browsegrades.net
Student Exploration Calorimetry Lab Vocabulary calorie, calorimeter Calorie Lab Answers You will use the calorimetry lab gizmotm to determine the specific heat capacities of various substances. Calorimeters can be used to find a substance’s specific heat capacity. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. When a hot object is placed in the calorimeter,. Calorie Lab Answers.
From www.studypool.com
SOLUTION 114 lab calorie counting worksheet Studypool Calorie Lab Answers This process is the basis of the technique. You will use the calorimetry lab gizmotm to determine the specific heat capacities of various substances. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. • read the lab thoroughly. Calorimeters can be used to find a. Calorie Lab Answers.
From www.scribd.com
Caloric Content of Food Lab Calorie Food Energy Calorie Lab Answers 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. Calorimeters can be used to find a substance’s specific heat capacity. A calorie is the amount of heat (energy) required to increase the temperature of 1 gram of water by 1 ° c. When a hot object is placed in the calorimeter,. Calorie Lab Answers.
From www.chegg.com
Solved 2.22 LAB Expression for calories burned during Calorie Lab Answers When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. You will use the calorimetry lab gizmotm to determine the specific heat capacities of various substances. • read the lab thoroughly. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65. Calorie Lab Answers.
From www.chegg.com
Solved E. Heat of Combustion and the Caloric Content of Food Calorie Lab Answers 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. This process is the basis of the technique. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. A calorie is the amount of heat (energy) required to. Calorie Lab Answers.
From www.studocu.com
Biofpx1000 assessment 4 Digestion Lab Question Caloric Need Answer Calorie Lab Answers • read the lab thoroughly. A calorie is the amount of heat (energy) required to increase the temperature of 1 gram of water by 1 ° c. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. 1) if 0.315 moles of hexane (c6h14) is combusted. Calorie Lab Answers.
From www.studocu.com
Calories Lab notes Purpose To determine the caloric content of Calorie Lab Answers 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. This process is the basis of the technique. • read the lab thoroughly. Calorimeters can be used to find a substance’s specific heat capacity. A calorie is the amount of heat (energy) required to increase the temperature of 1 gram of water. Calorie Lab Answers.
From www.coursehero.com
[Solved] 1.22 LAB Expression for calories burned during workout E Z 1 Calorie Lab Answers 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. A calorie is the amount of heat (energy) required to increase the temperature of 1 gram of water by 1 ° c. This process is the basis of the technique. You will use the calorimetry lab gizmotm to determine the specific heat. Calorie Lab Answers.