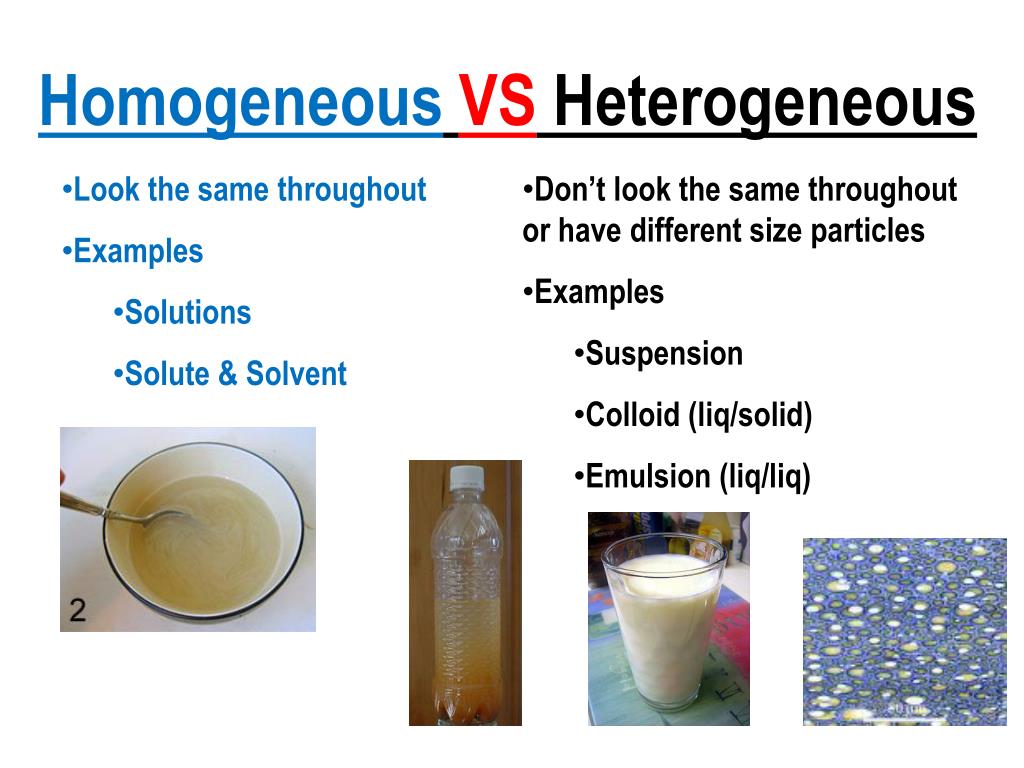
Is Foil Homogeneous Or Heterogeneous . Hence, aluminum is a pure substance. However, when aluminum foil is rolled up and bent, it takes on a new form. A homogeneous mixture, also known as a solution, contains particles that are very small. mixtures can be classified as homogeneous or heterogeneous. Aluminum foil is a heterogeneous mixture. explain the difference between a homogeneous mixture and a heterogeneous mixture. Matter can also be classified as a mixture. Elements and compounds are both examples of pure. Mixtures can either be homogeneous or heterogeneous. But what if my sample has several different types of elements and compounds all mixed together? Physical or chemical processes cannot further degrade them into new material. by definition, a pure substance or a homogeneous mixture consists of a single phase. is aluminum foil a heterogeneous mixture or a homogeneous mixture? Matter is anything that has mass and volume. we have found that aluminum foil is generally homogeneous, although certain types of foil and heating processes can lead to heterogeneous.
from www.slideserve.com
by definition, a pure substance or a homogeneous mixture consists of a single phase. Elements and compounds are both examples of pure. it has a uniform composition and is homogeneous. Aluminum foil is a heterogeneous mixture. Mixtures can either be homogeneous or heterogeneous. Matter is anything that has mass and volume. explain the difference between a homogeneous mixture and a heterogeneous mixture. we have found that aluminum foil is generally homogeneous, although certain types of foil and heating processes can lead to heterogeneous. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. However, when aluminum foil is rolled up and bent, it takes on a new form.
PPT Topics States of Matter Pure Substances Mixtures Physical and Chemical Changes Law of
Is Foil Homogeneous Or Heterogeneous But what if my sample has several different types of elements and compounds all mixed together? Physical or chemical processes cannot further degrade them into new material. explain the difference between a homogeneous mixture and a heterogeneous mixture. Matter can also be classified as a mixture. A homogeneous mixture, also known as a solution, contains particles that are very small. But what if my sample has several different types of elements and compounds all mixed together? is aluminum foil a heterogeneous mixture or a homogeneous mixture? mixtures can be classified as homogeneous or heterogeneous. Hence, aluminum is a pure substance. by definition, a pure substance or a homogeneous mixture consists of a single phase. Elements and compounds are both examples of pure. like real gold and fool’s gold. Mixtures can either be homogeneous or heterogeneous. it has a uniform composition and is homogeneous. we have found that aluminum foil is generally homogeneous, although certain types of foil and heating processes can lead to heterogeneous. Matter is anything that has mass and volume.
From www.pdfprof.com
is aluminum foil homogeneous or heterogeneous Is Foil Homogeneous Or Heterogeneous But what if my sample has several different types of elements and compounds all mixed together? Elements and compounds are both examples of pure. A heterogeneous mixture consists of two or more. However, when aluminum foil is rolled up and bent, it takes on a new form. Matter can also be classified as a mixture. Hence, aluminum is a pure. Is Foil Homogeneous Or Heterogeneous.
From pdfprof.com
is aluminum foil homogeneous or heterogeneous Is Foil Homogeneous Or Heterogeneous However, when aluminum foil is rolled up and bent, it takes on a new form. Matter is anything that has mass and volume. A heterogeneous mixture consists of two or more. is aluminum foil a heterogeneous mixture or a homogeneous mixture? But what if my sample has several different types of elements and compounds all mixed together? Aluminum foil. Is Foil Homogeneous Or Heterogeneous.
From slideplayer.com
Heterogeneous vs. Homogeneous ppt download Is Foil Homogeneous Or Heterogeneous Aluminum foil is a heterogeneous mixture. like real gold and fool’s gold. by definition, a pure substance or a homogeneous mixture consists of a single phase. explain the difference between a homogeneous mixture and a heterogeneous mixture. we have found that aluminum foil is generally homogeneous, although certain types of foil and heating processes can lead. Is Foil Homogeneous Or Heterogeneous.
From pdfprof.com
is aluminum foil homogeneous or heterogeneous Is Foil Homogeneous Or Heterogeneous But what if my sample has several different types of elements and compounds all mixed together? mixtures can be classified as homogeneous or heterogeneous. Mixtures can either be homogeneous or heterogeneous. Matter can also be classified as a mixture. we have found that aluminum foil is generally homogeneous, although certain types of foil and heating processes can lead. Is Foil Homogeneous Or Heterogeneous.
From www.slideserve.com
PPT Topics States of Matter Pure Substances Mixtures Physical and Chemical Changes Law of Is Foil Homogeneous Or Heterogeneous it has a uniform composition and is homogeneous. mixtures can be classified as homogeneous or heterogeneous. Physical or chemical processes cannot further degrade them into new material. Matter can also be classified as a mixture. Mixtures can either be homogeneous or heterogeneous. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. A. Is Foil Homogeneous Or Heterogeneous.
From www.pdfprof.com
is aluminum foil a homogeneous or heterogeneous mixture Is Foil Homogeneous Or Heterogeneous we have found that aluminum foil is generally homogeneous, although certain types of foil and heating processes can lead to heterogeneous. mixtures can be classified as homogeneous or heterogeneous. A homogeneous mixture, also known as a solution, contains particles that are very small. Aluminum foil is a heterogeneous mixture. Matter can also be classified as a mixture. But. Is Foil Homogeneous Or Heterogeneous.
From www.slideserve.com
PPT Unit 2 Nature of Matter and Theory PowerPoint Presentation ID685773 Is Foil Homogeneous Or Heterogeneous mixtures can be classified as homogeneous or heterogeneous. Elements and compounds are both examples of pure. Matter can also be classified as a mixture. Physical or chemical processes cannot further degrade them into new material. However, when aluminum foil is rolled up and bent, it takes on a new form. is aluminum foil a heterogeneous mixture or a. Is Foil Homogeneous Or Heterogeneous.
From www.slideserve.com
PPT PSc.2.1 OBJECTIVE Understand types, properties and structure of matter. PowerPoint Is Foil Homogeneous Or Heterogeneous Physical or chemical processes cannot further degrade them into new material. Aluminum foil is a heterogeneous mixture. Hence, aluminum is a pure substance. Matter is anything that has mass and volume. is aluminum foil a heterogeneous mixture or a homogeneous mixture? explain the difference between a homogeneous mixture and a heterogeneous mixture. A heterogeneous mixture consists of two. Is Foil Homogeneous Or Heterogeneous.
From pdfprof.com
is aluminum foil homogeneous or heterogeneous Is Foil Homogeneous Or Heterogeneous by definition, a pure substance or a homogeneous mixture consists of a single phase. is aluminum foil a heterogeneous mixture or a homogeneous mixture? Physical or chemical processes cannot further degrade them into new material. we have found that aluminum foil is generally homogeneous, although certain types of foil and heating processes can lead to heterogeneous. Mixtures. Is Foil Homogeneous Or Heterogeneous.
From studylib.net
classifyingmatterworksheet1 Is Foil Homogeneous Or Heterogeneous But what if my sample has several different types of elements and compounds all mixed together? A heterogeneous mixture consists of two or more. Matter is anything that has mass and volume. Physical or chemical processes cannot further degrade them into new material. A homogeneous mixture, also known as a solution, contains particles that are very small. by definition,. Is Foil Homogeneous Or Heterogeneous.
From www.slideserve.com
PPT Unit 2 Nature of Matter and Theory PowerPoint Presentation ID685773 Is Foil Homogeneous Or Heterogeneous Physical or chemical processes cannot further degrade them into new material. Matter can also be classified as a mixture. Mixtures can either be homogeneous or heterogeneous. But what if my sample has several different types of elements and compounds all mixed together? A heterogeneous mixture consists of two or more. is aluminum foil a heterogeneous mixture or a homogeneous. Is Foil Homogeneous Or Heterogeneous.
From www.museoinclusivo.com
Is Aluminum Foil Homogeneous or Heterogeneous? Exploring its Physical, Chemical, Structural and Is Foil Homogeneous Or Heterogeneous A homogeneous mixture, also known as a solution, contains particles that are very small. A heterogeneous mixture consists of two or more. is aluminum foil a heterogeneous mixture or a homogeneous mixture? Hence, aluminum is a pure substance. like real gold and fool’s gold. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous. Is Foil Homogeneous Or Heterogeneous.
From www.thoughtco.com
Heterogeneous vs. Homogeneous Mixtures Is Foil Homogeneous Or Heterogeneous A heterogeneous mixture consists of two or more. A homogeneous mixture, also known as a solution, contains particles that are very small. But what if my sample has several different types of elements and compounds all mixed together? Physical or chemical processes cannot further degrade them into new material. is aluminum foil a heterogeneous mixture or a homogeneous mixture?. Is Foil Homogeneous Or Heterogeneous.
From pdfprof.com
is aluminum foil homogeneous or heterogeneous Is Foil Homogeneous Or Heterogeneous However, when aluminum foil is rolled up and bent, it takes on a new form. Elements and compounds are both examples of pure. But what if my sample has several different types of elements and compounds all mixed together? we have found that aluminum foil is generally homogeneous, although certain types of foil and heating processes can lead to. Is Foil Homogeneous Or Heterogeneous.
From slideplayer.com
Review Game Chapters 1 & 2 Begin Game. ppt download Is Foil Homogeneous Or Heterogeneous Matter can also be classified as a mixture. Elements and compounds are both examples of pure. Hence, aluminum is a pure substance. we have found that aluminum foil is generally homogeneous, although certain types of foil and heating processes can lead to heterogeneous. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. Physical. Is Foil Homogeneous Or Heterogeneous.
From www.teachoo.com
Homogeneous and Hetrogeneous Mixtures Definition, Examples Teachoo Is Foil Homogeneous Or Heterogeneous Physical or chemical processes cannot further degrade them into new material. But what if my sample has several different types of elements and compounds all mixed together? Elements and compounds are both examples of pure. mixtures can be classified as homogeneous or heterogeneous. Mixtures can either be homogeneous or heterogeneous. Matter is anything that has mass and volume. . Is Foil Homogeneous Or Heterogeneous.
From www.etutorworld.com
Heterogenous & Homogenous Mixtures Key Differences, Definition, FAQs Is Foil Homogeneous Or Heterogeneous But what if my sample has several different types of elements and compounds all mixed together? it has a uniform composition and is homogeneous. Mixtures can either be homogeneous or heterogeneous. is aluminum foil a heterogeneous mixture or a homogeneous mixture? Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. A heterogeneous. Is Foil Homogeneous Or Heterogeneous.
From www.pdfprof.com
is aluminum foil a homogeneous or heterogeneous mixture Is Foil Homogeneous Or Heterogeneous Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. like real gold and fool’s gold. Elements and compounds are both examples of pure. Mixtures can either be homogeneous or heterogeneous. A homogeneous mixture, also known as a solution, contains particles that are very small. Hence, aluminum is a pure substance. Physical or chemical. Is Foil Homogeneous Or Heterogeneous.
From pdfprof.com
is aluminum foil homogeneous or heterogeneous Is Foil Homogeneous Or Heterogeneous Physical or chemical processes cannot further degrade them into new material. Matter is anything that has mass and volume. is aluminum foil a heterogeneous mixture or a homogeneous mixture? However, when aluminum foil is rolled up and bent, it takes on a new form. A homogeneous mixture, also known as a solution, contains particles that are very small. . Is Foil Homogeneous Or Heterogeneous.
From themasterchemistry.com
15 Examples Of Homogeneous Mixtures Is Foil Homogeneous Or Heterogeneous Physical or chemical processes cannot further degrade them into new material. Matter can also be classified as a mixture. we have found that aluminum foil is generally homogeneous, although certain types of foil and heating processes can lead to heterogeneous. Aluminum foil is a heterogeneous mixture. is aluminum foil a heterogeneous mixture or a homogeneous mixture? A homogeneous. Is Foil Homogeneous Or Heterogeneous.
From slideplayer.com
Chapter 2 Antacids. ppt download Is Foil Homogeneous Or Heterogeneous Mixtures can either be homogeneous or heterogeneous. mixtures can be classified as homogeneous or heterogeneous. Matter can also be classified as a mixture. it has a uniform composition and is homogeneous. However, when aluminum foil is rolled up and bent, it takes on a new form. explain the difference between a homogeneous mixture and a heterogeneous mixture.. Is Foil Homogeneous Or Heterogeneous.
From kitchenouse.com
Is Aluminum Foil A Homogeneous Mixture Is Foil Homogeneous Or Heterogeneous Physical or chemical processes cannot further degrade them into new material. it has a uniform composition and is homogeneous. explain the difference between a homogeneous mixture and a heterogeneous mixture. But what if my sample has several different types of elements and compounds all mixed together? Matter can also be classified as a mixture. by definition, a. Is Foil Homogeneous Or Heterogeneous.
From aluminumfoilnitoriru.blogspot.com
Aluminum Foil Aluminum Foil Homogeneous Or Heterogeneous Is Foil Homogeneous Or Heterogeneous A heterogeneous mixture consists of two or more. Matter can also be classified as a mixture. Hence, aluminum is a pure substance. like real gold and fool’s gold. explain the difference between a homogeneous mixture and a heterogeneous mixture. we have found that aluminum foil is generally homogeneous, although certain types of foil and heating processes can. Is Foil Homogeneous Or Heterogeneous.
From pdfprof.com
is aluminum foil homogeneous or heterogeneous Is Foil Homogeneous Or Heterogeneous Matter is anything that has mass and volume. like real gold and fool’s gold. A heterogeneous mixture consists of two or more. A homogeneous mixture, also known as a solution, contains particles that are very small. Aluminum foil is a heterogeneous mixture. Mixtures can either be homogeneous or heterogeneous. by definition, a pure substance or a homogeneous mixture. Is Foil Homogeneous Or Heterogeneous.
From www.slideserve.com
PPT Classify the following mixtures as either homogeneous or heterogeneous. PowerPoint Is Foil Homogeneous Or Heterogeneous Matter is anything that has mass and volume. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. is aluminum foil a heterogeneous mixture or a homogeneous mixture? Matter can also be classified as a mixture. Elements and compounds are both examples of pure. like real gold and fool’s gold. Hence, aluminum is. Is Foil Homogeneous Or Heterogeneous.
From printablelistquinta.z21.web.core.windows.net
Homogeneous And Heterogeneous Lesson Plan Is Foil Homogeneous Or Heterogeneous is aluminum foil a heterogeneous mixture or a homogeneous mixture? Matter can also be classified as a mixture. Mixtures can either be homogeneous or heterogeneous. explain the difference between a homogeneous mixture and a heterogeneous mixture. A heterogeneous mixture consists of two or more. by definition, a pure substance or a homogeneous mixture consists of a single. Is Foil Homogeneous Or Heterogeneous.
From adlanwachee.blogspot.com
Differences Between Heterogeneous And Homogeneous Mixture Is Foil Homogeneous Or Heterogeneous by definition, a pure substance or a homogeneous mixture consists of a single phase. is aluminum foil a heterogeneous mixture or a homogeneous mixture? like real gold and fool’s gold. mixtures can be classified as homogeneous or heterogeneous. Elements and compounds are both examples of pure. explain the difference between a homogeneous mixture and a. Is Foil Homogeneous Or Heterogeneous.
From byjus.com
explain although air is a homogeneous mixture within troposheric limits but in the atmospheric Is Foil Homogeneous Or Heterogeneous like real gold and fool’s gold. Matter can also be classified as a mixture. Physical or chemical processes cannot further degrade them into new material. is aluminum foil a heterogeneous mixture or a homogeneous mixture? Matter is anything that has mass and volume. Hence, aluminum is a pure substance. mixtures can be classified as homogeneous or heterogeneous.. Is Foil Homogeneous Or Heterogeneous.
From pdfprof.com
is aluminum foil homogeneous or heterogeneous Is Foil Homogeneous Or Heterogeneous Mixtures can either be homogeneous or heterogeneous. Aluminum foil is a heterogeneous mixture. A homogeneous mixture, also known as a solution, contains particles that are very small. A heterogeneous mixture consists of two or more. However, when aluminum foil is rolled up and bent, it takes on a new form. explain the difference between a homogeneous mixture and a. Is Foil Homogeneous Or Heterogeneous.
From kitchenouse.com
Is Aluminum Foil A Homogeneous Mixture Is Foil Homogeneous Or Heterogeneous But what if my sample has several different types of elements and compounds all mixed together? Physical or chemical processes cannot further degrade them into new material. Elements and compounds are both examples of pure. Aluminum foil is a heterogeneous mixture. However, when aluminum foil is rolled up and bent, it takes on a new form. Hence, aluminum is a. Is Foil Homogeneous Or Heterogeneous.
From ar.inspiredpencil.com
Heterogeneous Mixture Vs Homogeneous Mixture Is Foil Homogeneous Or Heterogeneous by definition, a pure substance or a homogeneous mixture consists of a single phase. Mixtures can either be homogeneous or heterogeneous. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. mixtures can be classified as homogeneous or heterogeneous. Matter can also be classified as a mixture. A heterogeneous mixture consists of two. Is Foil Homogeneous Or Heterogeneous.
From kitchenouse.com
Is Aluminum Foil A Homogeneous Mixture Is Foil Homogeneous Or Heterogeneous Elements and compounds are both examples of pure. But what if my sample has several different types of elements and compounds all mixed together? explain the difference between a homogeneous mixture and a heterogeneous mixture. Mixtures can either be homogeneous or heterogeneous. Physical or chemical processes cannot further degrade them into new material. Hence, aluminum is a pure substance.. Is Foil Homogeneous Or Heterogeneous.
From www.youtube.com
Is air a homogeneous or heterogeneous mixture? QnA Explained YouTube Is Foil Homogeneous Or Heterogeneous Mixtures can either be homogeneous or heterogeneous. Elements and compounds are both examples of pure. explain the difference between a homogeneous mixture and a heterogeneous mixture. Aluminum foil is a heterogeneous mixture. A heterogeneous mixture consists of two or more. by definition, a pure substance or a homogeneous mixture consists of a single phase. Physical or chemical processes. Is Foil Homogeneous Or Heterogeneous.
From pdfprof.com
is aluminum foil homogeneous or heterogeneous Is Foil Homogeneous Or Heterogeneous Elements and compounds are both examples of pure. Mixtures can either be homogeneous or heterogeneous. by definition, a pure substance or a homogeneous mixture consists of a single phase. However, when aluminum foil is rolled up and bent, it takes on a new form. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture.. Is Foil Homogeneous Or Heterogeneous.
From byjus.com
Homogenous is a type of mixed substance and how compound is homogenous if it is , then give me Is Foil Homogeneous Or Heterogeneous by definition, a pure substance or a homogeneous mixture consists of a single phase. Matter can also be classified as a mixture. Physical or chemical processes cannot further degrade them into new material. Hence, aluminum is a pure substance. Elements and compounds are both examples of pure. However, when aluminum foil is rolled up and bent, it takes on. Is Foil Homogeneous Or Heterogeneous.