Mace Clinical Endpoint . upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or procedures. 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical trials and cardiovascular research and can. components of mace endpoints and diagnostic codes used varied widely across observational studies. our analysis shows that mace remains one of the strongest adverse outcomes among stemi patients.
from www.cureus.com
22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical trials and cardiovascular research and can. components of mace endpoints and diagnostic codes used varied widely across observational studies. our analysis shows that mace remains one of the strongest adverse outcomes among stemi patients. upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or procedures.
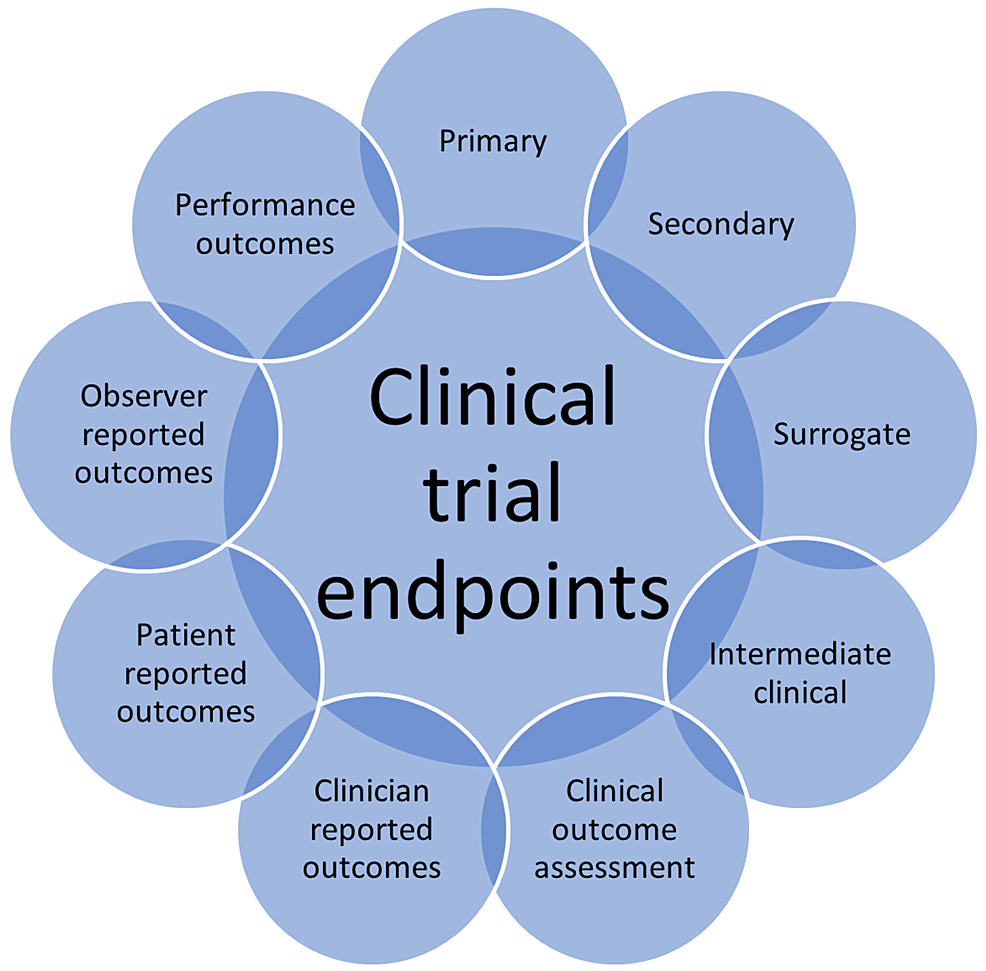
Cureus Research Question, Objectives, and Endpoints in Clinical and Oncological Research A
Mace Clinical Endpoint upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or procedures. upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or procedures. components of mace endpoints and diagnostic codes used varied widely across observational studies. our analysis shows that mace remains one of the strongest adverse outcomes among stemi patients. 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical trials and cardiovascular research and can.
From www.researchgate.net
Freedom from primary endpoint at 376 ± 18.1 days of followup (MACE... Download Scientific Mace Clinical Endpoint 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical trials and cardiovascular research and can. our analysis shows that mace remains one of the strongest adverse outcomes among stemi patients. upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence. Mace Clinical Endpoint.
From www.researchgate.net
Unadjusted and multivariateadjusted survival plot of composite... Download Scientific Diagram Mace Clinical Endpoint components of mace endpoints and diagnostic codes used varied widely across observational studies. upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or procedures. 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical. Mace Clinical Endpoint.
From www.researchgate.net
KaplanMeier curve for major adverse cardiac events (MACE). Clinical... Download Scientific Mace Clinical Endpoint components of mace endpoints and diagnostic codes used varied widely across observational studies. our analysis shows that mace remains one of the strongest adverse outcomes among stemi patients. upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or. Mace Clinical Endpoint.
From slidetodoc.com
Effect of BET Protein Inhibition With Apabetalone on Mace Clinical Endpoint our analysis shows that mace remains one of the strongest adverse outcomes among stemi patients. components of mace endpoints and diagnostic codes used varied widely across observational studies. upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or. Mace Clinical Endpoint.
From www.slideserve.com
PPT Multiple Primary Endpoints in Clinical Trials PowerPoint Presentation ID6961869 Mace Clinical Endpoint 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical trials and cardiovascular research and can. components of mace endpoints and diagnostic codes used varied widely across observational studies. upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization. Mace Clinical Endpoint.
From slideplayer.com
Background The clinical impact of lipidrich plaque (LRP) in patients with coronary Mace Clinical Endpoint components of mace endpoints and diagnostic codes used varied widely across observational studies. upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or procedures. 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical. Mace Clinical Endpoint.
From www.researchgate.net
Definition of study endpoints, MACE, and adjusted confounders in the... Download Scientific Mace Clinical Endpoint our analysis shows that mace remains one of the strongest adverse outcomes among stemi patients. components of mace endpoints and diagnostic codes used varied widely across observational studies. upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or. Mace Clinical Endpoint.
From www.researchgate.net
(PDF) Statistical power for MACE and individual secondary endpoints in cardiovascular Mace Clinical Endpoint components of mace endpoints and diagnostic codes used varied widely across observational studies. upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or procedures. our analysis shows that mace remains one of the strongest adverse outcomes among stemi. Mace Clinical Endpoint.
From www.researchgate.net
KaplanMeier curve for major adverse cardiac events (MACE). Clinical... Download Scientific Mace Clinical Endpoint our analysis shows that mace remains one of the strongest adverse outcomes among stemi patients. components of mace endpoints and diagnostic codes used varied widely across observational studies. 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical trials and cardiovascular research and can. upon sensitivity analysis, our alternative mace end point. Mace Clinical Endpoint.
From www.researchgate.net
Clinical Presentation of MACE in Lung Cancer Patients Receiving ICI Download Scientific Diagram Mace Clinical Endpoint upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or procedures. our analysis shows that mace remains one of the strongest adverse outcomes among stemi patients. components of mace endpoints and diagnostic codes used varied widely across observational. Mace Clinical Endpoint.
From www.endpointclinical.com
Solutions endpoint Clinical Mace Clinical Endpoint upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or procedures. components of mace endpoints and diagnostic codes used varied widely across observational studies. 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical. Mace Clinical Endpoint.
From slideplayer.com
Angioplasty Balloonassociated Coronary Debris and the EZ FilterWire ppt download Mace Clinical Endpoint components of mace endpoints and diagnostic codes used varied widely across observational studies. upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or procedures. our analysis shows that mace remains one of the strongest adverse outcomes among stemi. Mace Clinical Endpoint.
From www.researchgate.net
Results of cardiovascular studies comparing GLP1 RAs with... Download Scientific Diagram Mace Clinical Endpoint components of mace endpoints and diagnostic codes used varied widely across observational studies. our analysis shows that mace remains one of the strongest adverse outcomes among stemi patients. upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or. Mace Clinical Endpoint.
From www.researchgate.net
Regression of LLL, MLD, or DS vs. clinical endpoints (TLR, TLF, and... Download Scientific Mace Clinical Endpoint upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or procedures. components of mace endpoints and diagnostic codes used varied widely across observational studies. 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical. Mace Clinical Endpoint.
From www.opportunityindia.com
Endpoint Clinical opens new office in Hyderabad Mace Clinical Endpoint upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or procedures. 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical trials and cardiovascular research and can. components of mace endpoints and diagnostic codes. Mace Clinical Endpoint.
From www.researchgate.net
Association between baseline COPD and clinical This forest... Download Scientific Mace Clinical Endpoint 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical trials and cardiovascular research and can. components of mace endpoints and diagnostic codes used varied widely across observational studies. our analysis shows that mace remains one of the strongest adverse outcomes among stemi patients. upon sensitivity analysis, our alternative mace end point. Mace Clinical Endpoint.
From slideplayer.com
ENDEAVOR III Multicenter Randomized Trial Clinical/MACE Angio/IVUS ppt download Mace Clinical Endpoint 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical trials and cardiovascular research and can. components of mace endpoints and diagnostic codes used varied widely across observational studies. our analysis shows that mace remains one of the strongest adverse outcomes among stemi patients. upon sensitivity analysis, our alternative mace end point. Mace Clinical Endpoint.
From www.cureus.com
Cureus Research Question, Objectives, and Endpoints in Clinical and Oncological Research A Mace Clinical Endpoint upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or procedures. 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical trials and cardiovascular research and can. components of mace endpoints and diagnostic codes. Mace Clinical Endpoint.
From www.tldrpharmacy.com
A Guide to Clinical Trial Endpoints — tl;dr pharmacy Mace Clinical Endpoint our analysis shows that mace remains one of the strongest adverse outcomes among stemi patients. 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical trials and cardiovascular research and can. upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence. Mace Clinical Endpoint.
From slideplayer.com
Professor Ken McDonald National Clinical Lead for Heart Failure and ppt download Mace Clinical Endpoint components of mace endpoints and diagnostic codes used varied widely across observational studies. upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or procedures. 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical. Mace Clinical Endpoint.
From www.researchgate.net
Clinical endpoints. Download Table Mace Clinical Endpoint components of mace endpoints and diagnostic codes used varied widely across observational studies. 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical trials and cardiovascular research and can. upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization. Mace Clinical Endpoint.
From tools4.us
Tools4us Endpoint Clinical Mace Clinical Endpoint our analysis shows that mace remains one of the strongest adverse outcomes among stemi patients. components of mace endpoints and diagnostic codes used varied widely across observational studies. upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or. Mace Clinical Endpoint.
From www.gastrojournal.org
Identification of Endpoints for Development of Antifibrosis Drugs for Treatment of Crohn’s Mace Clinical Endpoint components of mace endpoints and diagnostic codes used varied widely across observational studies. our analysis shows that mace remains one of the strongest adverse outcomes among stemi patients. 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical trials and cardiovascular research and can. upon sensitivity analysis, our alternative mace end point. Mace Clinical Endpoint.
From www.bostonscientific.com
Chronic Total Occlusion (CTO) System Clinical Data Boston Scientific Mace Clinical Endpoint 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical trials and cardiovascular research and can. our analysis shows that mace remains one of the strongest adverse outcomes among stemi patients. components of mace endpoints and diagnostic codes used varied widely across observational studies. upon sensitivity analysis, our alternative mace end point. Mace Clinical Endpoint.
From www.ahajournals.org
Composite End Points in Clinical Research Circulation Mace Clinical Endpoint components of mace endpoints and diagnostic codes used varied widely across observational studies. upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or procedures. 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical. Mace Clinical Endpoint.
From www.researchgate.net
Forest plots depicting clinical endpoints of P2Y12 deescalation... Download Scientific Diagram Mace Clinical Endpoint our analysis shows that mace remains one of the strongest adverse outcomes among stemi patients. upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or procedures. components of mace endpoints and diagnostic codes used varied widely across observational. Mace Clinical Endpoint.
From slideplayer.com
Hexabrix Key Clinical Review Le Feuvre, ppt download Mace Clinical Endpoint upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or procedures. components of mace endpoints and diagnostic codes used varied widely across observational studies. our analysis shows that mace remains one of the strongest adverse outcomes among stemi. Mace Clinical Endpoint.
From www.researchgate.net
Overview of the MACE framework. Download Scientific Diagram Mace Clinical Endpoint 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical trials and cardiovascular research and can. upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or procedures. components of mace endpoints and diagnostic codes. Mace Clinical Endpoint.
From www.tldrpharmacy.com
A Guide to Clinical Trial Endpoints — tl;dr pharmacy Mace Clinical Endpoint upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or procedures. our analysis shows that mace remains one of the strongest adverse outcomes among stemi patients. components of mace endpoints and diagnostic codes used varied widely across observational. Mace Clinical Endpoint.
From www.researchgate.net
Freedom from primary endpoint at 376 ± 18.1 days of followup (MACE... Download Scientific Mace Clinical Endpoint components of mace endpoints and diagnostic codes used varied widely across observational studies. 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical trials and cardiovascular research and can. our analysis shows that mace remains one of the strongest adverse outcomes among stemi patients. upon sensitivity analysis, our alternative mace end point. Mace Clinical Endpoint.
From www.researchgate.net
(PDF) Retrospective Study of Appropriate Primary Prevention in Postmenopausal Women Presenting Mace Clinical Endpoint 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical trials and cardiovascular research and can. components of mace endpoints and diagnostic codes used varied widely across observational studies. our analysis shows that mace remains one of the strongest adverse outcomes among stemi patients. upon sensitivity analysis, our alternative mace end point. Mace Clinical Endpoint.
From www.nature.com
Statistical power for MACE and individual secondary endpoints in cardiovascular trials Mace Clinical Endpoint 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical trials and cardiovascular research and can. our analysis shows that mace remains one of the strongest adverse outcomes among stemi patients. components of mace endpoints and diagnostic codes used varied widely across observational studies. upon sensitivity analysis, our alternative mace end point. Mace Clinical Endpoint.
From www.researchgate.net
Study flowchart; MACEmajor adverse cardiovascular event. Download Scientific Diagram Mace Clinical Endpoint 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical trials and cardiovascular research and can. upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or procedures. components of mace endpoints and diagnostic codes. Mace Clinical Endpoint.
From www.researchgate.net
Twoyear deviceoriented clinical endpoint and individual clinical... Download Scientific Diagram Mace Clinical Endpoint our analysis shows that mace remains one of the strongest adverse outcomes among stemi patients. components of mace endpoints and diagnostic codes used varied widely across observational studies. 22 rows mace refers to a clinical endpoint used for outcome evaluations in clinical trials and cardiovascular research and can. upon sensitivity analysis, our alternative mace end point. Mace Clinical Endpoint.
From slideplayer.com
The Elevated Role of GLP1 RAs in Diabetes Management Which Patients Should We Aim For? ppt Mace Clinical Endpoint upon sensitivity analysis, our alternative mace end point again found higher rates of mace because of a 14.4% (4.02 events/100 pys) incidence of hospitalization for additional vascular events or procedures. our analysis shows that mace remains one of the strongest adverse outcomes among stemi patients. 22 rows mace refers to a clinical endpoint used for outcome evaluations. Mace Clinical Endpoint.