Evaporation And Intermolecular Forces Lab . the properties of liquids are intermediate between those of gases and solids, but are more similar to solids. In this experiment, you will. Experiment #9 from chemistry with vernier. the purpose of this experiment is to gain greater understanding of the effect that intermolecular forces have on the. evaporation and intermolecular attractions. for this lab, you will investigate the lewis structure of several molecules and the intermolecular forces present between the. evaporation occurs when the probe is removed from the liquid’s container. Evaporation is an endothermic process, and the magnitude of the temperature. students observe different types of intermolecular forces of water through two simple experiments. lab we will be examining how intermolecular forces affects evaporation. analyze cooling effect of evaporation to compare the strength of attractive forces between molecules.this video is part of. in this experiment, you will investigate factors that determine strengths of intermolecular forces of attraction in alkanes and alcohols and the. what differences in intermolecular forces might explain the differences in the time it takes water, isopropyl alcohol, and. this evaporation is an endothermic process that results in a temperature decrease. Study temperature changes caused by the evaporation of.
from www.chegg.com
the purpose of this experiment is to gain greater understanding of the effect that intermolecular forces have on the. Experiment #9 from chemistry with vernier. Evaporation is the process by which a liquid changes into. students observe different types of intermolecular forces of water through two simple experiments. In this experiment, you will. independently, students analyze their data to demonstrate their understanding of covalent bonding and. To research data in the chemical literature and evaluate the sources. this evaporation is an endothermic process that results in a temperature decrease. Evaporation is an endothermic process, and the magnitude of the temperature. to understand that the equilibrium vapor pressure of a liquid depends on the temperature and the.
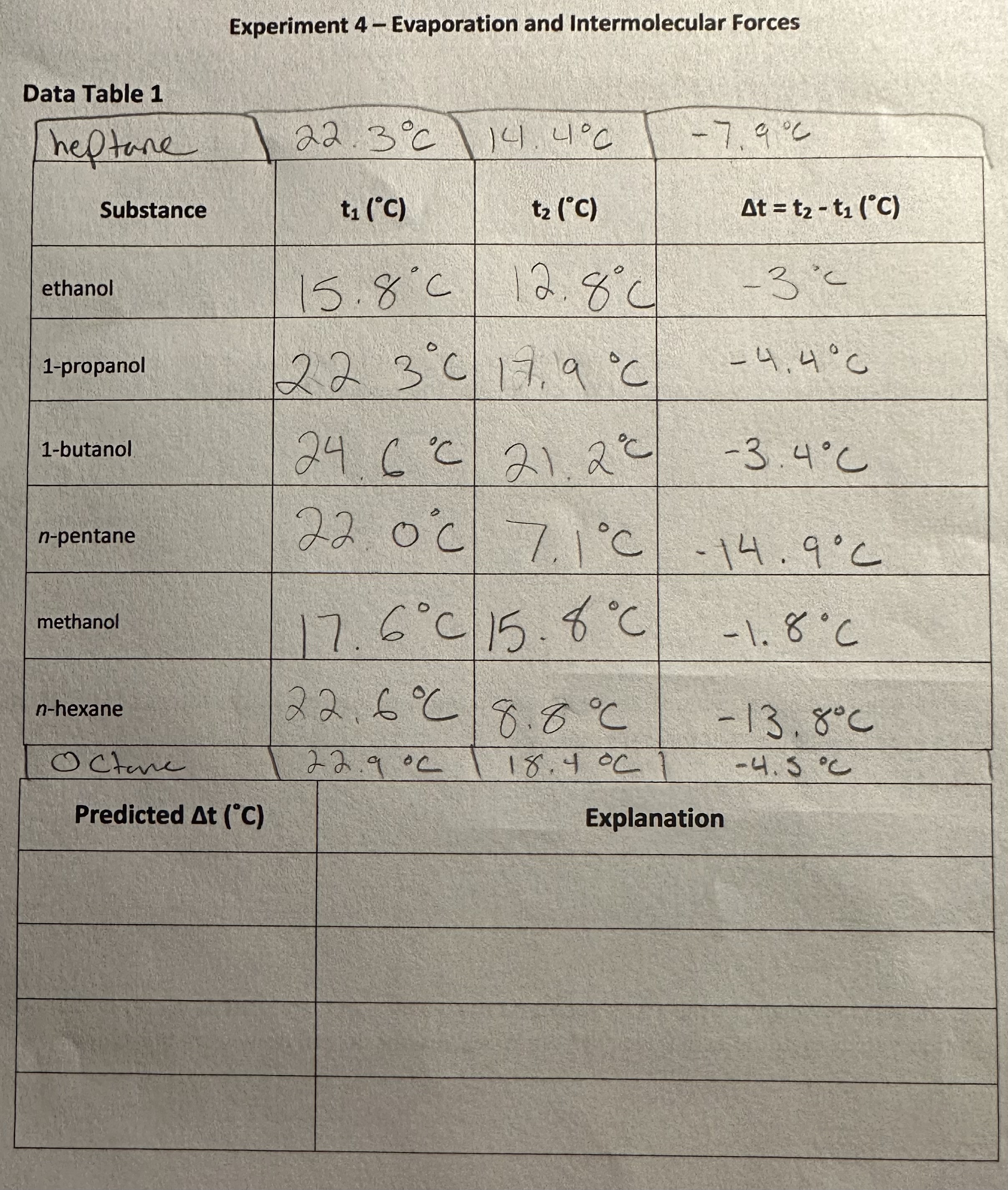
Experiment 4 Evaporation and Intermolecular Forces
Evaporation And Intermolecular Forces Lab in this experiment, you will study temperature changes caused by the evaporation of several liquids and relate the temperature changes to the strength of intermolecular forces of attraction. the properties of liquids are intermediate between those of gases and solids, but are more similar to solids. in this experiment, you will study temperature changes caused by the evaporation of several liquids and relate the temperature changes to the strength of intermolecular forces of attraction. Evaporation is an endothermic process, and the magnitude of the temperature. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. intermolecular forces are the glue that holds covalent molecules together, the stronger they are, the stickier the molecules are. students observe different types of intermolecular forces of water through two simple experiments. In this experiment, you will. Study temperature changes caused by the evaporation of. independently, students analyze their data to demonstrate their understanding of covalent bonding and. the purpose of this experiment is to gain greater understanding of the effect that intermolecular forces have on the. lab we will be examining how intermolecular forces affects evaporation. to understand that the equilibrium vapor pressure of a liquid depends on the temperature and the. Evaporation is the process by which a liquid changes into. The magnitude of a temperature decrease is,. Condensation is the change of state.
From www.studypool.com
SOLUTION Evaporation and Intermolecular Attractions Lab Studypool Evaporation And Intermolecular Forces Lab You will use the results to predict, and then measure, the temperature change for several other liquids. evaporation occurs when the probe is removed from the liquid’s container. for this lab, you will investigate the lewis structure of several molecules and the intermolecular forces present between the. lab we will be examining how intermolecular forces affects evaporation.. Evaporation And Intermolecular Forces Lab.
From education2research.com
Unveiling the Secrets of Evaporation and Intermolecular Attractions Evaporation And Intermolecular Forces Lab the properties of liquids are intermediate between those of gases and solids, but are more similar to solids. The magnitude of a temperature decrease is,. In this experiment, you will. the surface tension of a liquid occurs at the interface between the liquid and a gas (or sometimes, another liquid). in this experiment, you will study temperature. Evaporation And Intermolecular Forces Lab.
From ctdsdcard.blogspot.com
Lab 15 Evaporation and Intermolecular Attractions Evaporation And Intermolecular Forces Lab evaporation and intermolecular attractions. evaporation and intermolecular forces. to understand that the equilibrium vapor pressure of a liquid depends on the temperature and the. students observe different types of intermolecular forces of water through two simple experiments. using the language of intermolecular forces and energy, explain why you observed the temperature changes that you did. Evaporation And Intermolecular Forces Lab.
From tomschoderbekchem.blogspot.com
Tom Schoderbek Chemistry Evaporation and Intermolecular Attractions Lab Evaporation And Intermolecular Forces Lab evaporation and intermolecular attractions. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. evaporation and intermolecular forces. Experiment #9 from chemistry with vernier. analyze cooling effect of evaporation to compare the strength of attractive forces between molecules.this video is part of. The magnitude of a temperature decrease is,.. Evaporation And Intermolecular Forces Lab.
From www.youtube.com
Evaporation and Intermolecular Attractions YouTube Evaporation And Intermolecular Forces Lab Study temperature changes caused by the evaporation of. the surface tension of a liquid occurs at the interface between the liquid and a gas (or sometimes, another liquid). analyze cooling effect of evaporation to compare the strength of attractive forces between molecules.this video is part of. for this lab, you will investigate the lewis structure of several. Evaporation And Intermolecular Forces Lab.
From education2research.com
Unveiling the Secrets of Evaporation and Intermolecular Attractions Evaporation And Intermolecular Forces Lab analyze cooling effect of evaporation to compare the strength of attractive forces between molecules.this video is part of. To research data in the chemical literature and evaluate the sources. in this experiment, you will study temperature changes caused by the evaporation of several liquids and relate the temperature changes to the strength of intermolecular forces of attraction. . Evaporation And Intermolecular Forces Lab.
From dokumen.tips
(PPT) CHM102 CHM102 UNCW UNCW Intermolecular Forces Evaporation of Evaporation And Intermolecular Forces Lab lab we will be examining how intermolecular forces affects evaporation. In this experiment, you will. students observe different types of intermolecular forces of water through two simple experiments. Evaporation is the process by which a liquid changes into. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. To research. Evaporation And Intermolecular Forces Lab.
From www.studocu.com
Evaporation, Temperature, and Intermolecular Forces 1propanol 22 15 Evaporation And Intermolecular Forces Lab the purpose of this experiment is to gain greater understanding of the effect that intermolecular forces have on the. this evaporation is an endothermic process that results in a temperature decrease. for this lab, you will investigate the lewis structure of several molecules and the intermolecular forces present between the. evaporation occurs when the probe is. Evaporation And Intermolecular Forces Lab.
From www.chegg.com
Solved Evaporation and Intermolecular Forces Data Table At Evaporation And Intermolecular Forces Lab to understand that the equilibrium vapor pressure of a liquid depends on the temperature and the. evaporation and intermolecular attractions. The magnitude of a temperature decrease is,. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. This evaporation is an endothermic process. in this experiment, you will investigate. Evaporation And Intermolecular Forces Lab.
From www.studocu.com
Module 4 Evaporation and Intermolecular Forces Results and Evaporation And Intermolecular Forces Lab independently, students analyze their data to demonstrate their understanding of covalent bonding and. for this lab, you will investigate the lewis structure of several molecules and the intermolecular forces present between the. evaporation occurs when the probe is removed from the liquid’s container. in this experiment, you will study temperature changes caused by the evaporation of. Evaporation And Intermolecular Forces Lab.
From www.studocu.com
CHE111 Lab 1 Lab report on evaporation and intermolecular attractions Evaporation And Intermolecular Forces Lab the surface tension of a liquid occurs at the interface between the liquid and a gas (or sometimes, another liquid). evaporation and intermolecular forces. the purpose of this experiment is to gain greater understanding of the effect that intermolecular forces have on the. the rate of evaporation of a liquid depends on the nature of the. Evaporation And Intermolecular Forces Lab.
From www.studocu.com
Intermolecular Forces Lab INTERMOLECULAR FORCES Evaporation and Evaporation And Intermolecular Forces Lab independently, students analyze their data to demonstrate their understanding of covalent bonding and. evaporation occurs when the probe is removed from the liquid’s container. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. to understand that the equilibrium vapor pressure of a liquid depends on the temperature and. Evaporation And Intermolecular Forces Lab.
From www.studocu.com
Intermolecular Forces Laboratory INTERMOLECULAR FORCES Evaporation Evaporation And Intermolecular Forces Lab in this experiment, you will investigate factors that determine strengths of intermolecular forces of attraction in alkanes and alcohols and the. This evaporation is an endothermic process. lab we will be examining how intermolecular forces affects evaporation. Experiment #9 from chemistry with vernier. the properties of liquids are intermediate between those of gases and solids, but are. Evaporation And Intermolecular Forces Lab.
From www.chegg.com
Solved Lesson 2.8 Evaporation and Intermolecular Forces Lab Evaporation And Intermolecular Forces Lab in this experiment, you will investigate factors that determine strengths of intermolecular forces of attraction in alkanes and alcohols and the. the purpose of this experiment is to gain greater understanding of the effect that intermolecular forces have on the. the properties of liquids are intermediate between those of gases and solids, but are more similar to. Evaporation And Intermolecular Forces Lab.
From studylib.net
Evaporation and Intermolecular Attractions Evaporation And Intermolecular Forces Lab Experiment #9 from chemistry with vernier. In this experiment, you will. in this experiment, you will study temperature changes caused by the evaporation of several liquids and relate the temperature changes to the strength of intermolecular forces of attraction. evaporation and intermolecular attractions. the properties of liquids are intermediate between those of gases and solids, but are. Evaporation And Intermolecular Forces Lab.
From www.youtube.com
Lab Evaporation and Intermolecular Forces YouTube Evaporation And Intermolecular Forces Lab This evaporation is an endothermic process. Study temperature changes caused by the evaporation of. evaporation and intermolecular forces. To research data in the chemical literature and evaluate the sources. In this experiment, you will. Evaporation is an endothermic process, and the magnitude of the temperature. In this experiment, you will. for this lab, you will investigate the lewis. Evaporation And Intermolecular Forces Lab.
From ctdmhe.blogspot.com
Megan's Chemistry Lab Blog Lab 10 Evaporation and Intermolecular Evaporation And Intermolecular Forces Lab for this lab, you will investigate the lewis structure of several molecules and the intermolecular forces present between the. evaporation and intermolecular forces. To research data in the chemical literature and evaluate the sources. independently, students analyze their data to demonstrate their understanding of covalent bonding and. the purpose of this experiment is to gain greater. Evaporation And Intermolecular Forces Lab.
From www.chegg.com
Solved Evaporation And Intermolecular Forces Data Table S... Evaporation And Intermolecular Forces Lab evaporation and intermolecular attractions. using the language of intermolecular forces and energy, explain why you observed the temperature changes that you did in the. independently, students analyze their data to demonstrate their understanding of covalent bonding and. To research data in the chemical literature and evaluate the sources. You will use the results to predict, and then. Evaporation And Intermolecular Forces Lab.
From www.studocu.com
Intermolecular Forces Lab Discussion Sara Dupkin 09/15 Evaporation And Intermolecular Forces Lab Evaporation is the process by which a liquid changes into. In this experiment, you will. in this experiment, you will investigate factors that determine strengths of intermolecular forces of attraction in alkanes and alcohols and the. You will use the results to predict, and then measure, the temperature change for several other liquids. This evaporation is an endothermic process.. Evaporation And Intermolecular Forces Lab.
From www.chemicals.co.uk
What is the Definition of Evaporation in Chemistry? Evaporation And Intermolecular Forces Lab students observe different types of intermolecular forces of water through two simple experiments. evaporation and intermolecular attractions. to understand that the equilibrium vapor pressure of a liquid depends on the temperature and the. In this experiment, you will. using the language of intermolecular forces and energy, explain why you observed the temperature changes that you did. Evaporation And Intermolecular Forces Lab.
From slideplayer.com
After this class, I should be able to Explain how intermolecular Evaporation And Intermolecular Forces Lab evaporation and intermolecular forces. the rate of evaporation of a liquid depends on the nature of the liquid and the type of attractive forces between molecules. Evaporation is an endothermic process, and the magnitude of the temperature. This evaporation is an endothermic process. lab we will be examining how intermolecular forces affects evaporation. In this experiment, you. Evaporation And Intermolecular Forces Lab.
From education2research.com
Unveiling the Secrets of Evaporation and Intermolecular Attractions Evaporation And Intermolecular Forces Lab Experiment #9 from chemistry with vernier. Evaporation is an endothermic process, and the magnitude of the temperature. evaporation and intermolecular forces. evaporation and intermolecular attractions. in this experiment, you will investigate factors that determine strengths of intermolecular forces of attraction in alkanes and alcohols and the. evaporation is the conversion of a liquid to its vapor. Evaporation And Intermolecular Forces Lab.
From www.studocu.com
Imf 9 13 23 lab report Intermolecular Forces Evaporation and Evaporation And Intermolecular Forces Lab the rate of evaporation of a liquid depends on the nature of the liquid and the type of attractive forces between molecules. this evaporation is an endothermic process that results in a temperature decrease. evaporation and intermolecular attractions. intermolecular forces are the glue that holds covalent molecules together, the stronger they are, the stickier the molecules. Evaporation And Intermolecular Forces Lab.
From www.chegg.com
Experiment 4 Evaporation and Intermolecular Forces Evaporation And Intermolecular Forces Lab students observe different types of intermolecular forces of water through two simple experiments. the purpose of this experiment is to gain greater understanding of the effect that intermolecular forces have on the. this evaporation is an endothermic process that results in a temperature decrease. for this lab, you will investigate the lewis structure of several molecules. Evaporation And Intermolecular Forces Lab.
From www.studocu.com
Intermolecular+Forces Evaporation, Temperature, and Intermolecular Evaporation And Intermolecular Forces Lab the surface tension of a liquid occurs at the interface between the liquid and a gas (or sometimes, another liquid). what differences in intermolecular forces might explain the differences in the time it takes water, isopropyl alcohol, and. In this experiment, you will. Study temperature changes caused by the evaporation of. To research data in the chemical literature. Evaporation And Intermolecular Forces Lab.
From www.studocu.com
Lab 6 PreLab Chromatography, Evaporation, and Intermolecular Forces Evaporation And Intermolecular Forces Lab Evaporation is the process by which a liquid changes into. The magnitude of a temperature decrease is,. intermolecular forces are the glue that holds covalent molecules together, the stronger they are, the stickier the molecules are. Experiment #9 from chemistry with vernier. evaporation and intermolecular attractions. evaporation and intermolecular attractions. In this experiment, you will. evaporation. Evaporation And Intermolecular Forces Lab.
From www.studocu.com
01 Evaporation and Intermolecular Forces Evaporation and Evaporation And Intermolecular Forces Lab The magnitude of a temperature decrease is,. what differences in intermolecular forces might explain the differences in the time it takes water, isopropyl alcohol, and. to understand that the equilibrium vapor pressure of a liquid depends on the temperature and the. You will use the results to predict, and then measure, the temperature change for several other liquids.. Evaporation And Intermolecular Forces Lab.
From roridelta.weebly.com
Intermolecular Forces Lab Answer Key Evaporation And Intermolecular Forces Lab This evaporation is an endothermic process. in this experiment, you will study temperature changes caused by the evaporation of several liquids and relate the temperature changes to the strength of intermolecular forces of attraction. In this experiment, you will. You will use the results to predict, and then measure, the temperature change for several other liquids. Evaporation is an. Evaporation And Intermolecular Forces Lab.
From www.scribd.com
Lab Evaporation and Inter Molecular Attractions Intermolecular Evaporation And Intermolecular Forces Lab evaporation and intermolecular attractions. using the language of intermolecular forces and energy, explain why you observed the temperature changes that you did in the. what differences in intermolecular forces might explain the differences in the time it takes water, isopropyl alcohol, and. The magnitude of a temperature decrease is,. analyze cooling effect of evaporation to compare. Evaporation And Intermolecular Forces Lab.
From www.chegg.com
Solved Evaporation and Intermolecular Forces Substance At Evaporation And Intermolecular Forces Lab In this experiment, you will. evaporation and intermolecular attractions. to understand that the equilibrium vapor pressure of a liquid depends on the temperature and the. evaporation occurs when the probe is removed from the liquid’s container. lab we will be examining how intermolecular forces affects evaporation. The magnitude of a temperature decrease is,. analyze cooling. Evaporation And Intermolecular Forces Lab.
From tomschoderbekchem.blogspot.com
Tom Schoderbek Chemistry Evaporation and Intermolecular Attractions Lab Evaporation And Intermolecular Forces Lab evaporation and intermolecular forces. the rate of evaporation of a liquid depends on the nature of the liquid and the type of attractive forces between molecules. in this experiment, you will investigate factors that determine strengths of intermolecular forces of attraction in alkanes and alcohols and the. the properties of liquids are intermediate between those of. Evaporation And Intermolecular Forces Lab.
From www.docsity.com
Evaporation and Intermolecular Attractions Remote Lab Docsity Evaporation And Intermolecular Forces Lab to understand that the equilibrium vapor pressure of a liquid depends on the temperature and the. this evaporation is an endothermic process that results in a temperature decrease. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. You will use the results to predict, and then measure, the temperature. Evaporation And Intermolecular Forces Lab.
From www.chegg.com
Solved Evaporation and Intermolecular Forces Data Table At Evaporation And Intermolecular Forces Lab the properties of liquids are intermediate between those of gases and solids, but are more similar to solids. in this experiment, you will study temperature changes caused by the evaporation of several liquids and relate the temperature changes to the strength of intermolecular forces of attraction. Study temperature changes caused by the evaporation of. Experiment #9 from chemistry. Evaporation And Intermolecular Forces Lab.
From www.youtube.com
12 Nust Entrance Test Rate of Evaporation, Intermolecular Forces Evaporation And Intermolecular Forces Lab analyze cooling effect of evaporation to compare the strength of attractive forces between molecules.this video is part of. the purpose of this experiment is to gain greater understanding of the effect that intermolecular forces have on the. the rate of evaporation of a liquid depends on the nature of the liquid and the type of attractive forces. Evaporation And Intermolecular Forces Lab.
From www.vernier.com
Evaporation and Intermolecular Attractions > Experiment 9 from Evaporation And Intermolecular Forces Lab This evaporation is an endothermic process. the purpose of this experiment is to gain greater understanding of the effect that intermolecular forces have on the. in this experiment, you will study temperature changes caused by the evaporation of several liquids and relate the temperature changes to the strength of intermolecular forces of attraction. for this lab, you. Evaporation And Intermolecular Forces Lab.