Usp Test Method . read answers to frequently asked questions about usp's microbial enumeration of nonsterile products and more specifically,. assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation. the purpose of this chapter is to provide guidance for validating methods for use as alternatives to the official compendial. method validation ensures a test is reliable and reproducible. usp reference standards are authentic specimens that have been approved by the usp reference. What you need to know and how to find proven services. — product quality tests for topically applied drug products. Universal tests (see ich guidance. summary of usp container performance testing key points. the choice of a method is based on factors such as the nature of the product and the required limit of microorganisms. There are eight (8) different permeation methods. method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. This chapter describes analytical procedures for the evaluation of elemental impurities that are suitable for the limits described in. chemical tests and assays. — during early stages of method development, the robustness of methods should be.
from csanalytical.com
usp reference standards are authentic specimens that have been approved by the usp reference. — during early stages of method development, the robustness of methods should be. read answers to frequently asked questions about usp's microbial enumeration of nonsterile products and more specifically,. — the following are usp methods for pharmaceutical drugs: the types of chromatography useful in qualitative and quantitative analysis employed in usp procedures are column,. — product quality tests for topically applied drug products. the guidelines in usp general chapter <<strong>621</strong>> provide a clearer definition of permissible adjustments during method. already established general assays and tests (e.g., titrimetric method of water determination, bacterial endotoxins test). There are eight (8) different permeation methods. What you need to know and how to find proven services.
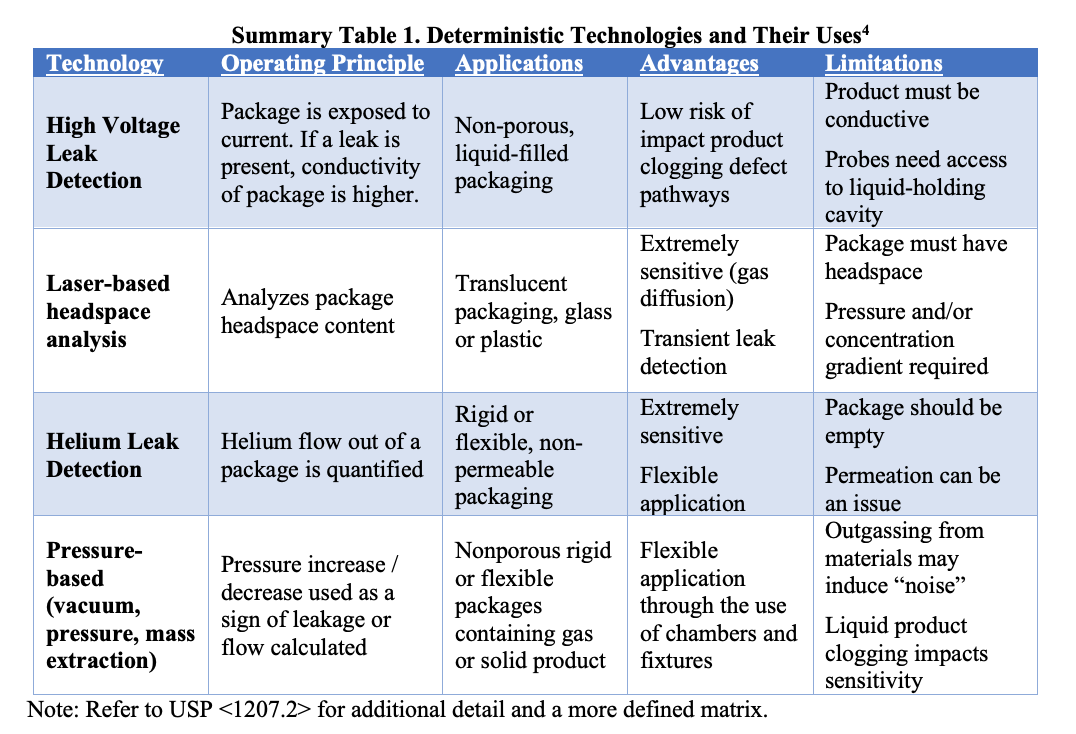
USP 1207 CCI for Combination Products
Usp Test Method the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. the guidelines in usp general chapter <<strong>621</strong>> provide a clearer definition of permissible adjustments during method. usp reference standards are authentic specimens that have been approved by the usp reference. — expert committee: read answers to frequently asked questions about usp's microbial enumeration of nonsterile products and more specifically,. method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. What you need to know and how to find proven services. the choice of a method is based on factors such as the nature of the product and the required limit of microorganisms. Á210ñ monosaccharide analysis (new), 8059 á232ñ elemental impurities—limits, 8065 introduction, routes of. the types of chromatography useful in qualitative and quantitative analysis employed in usp procedures are column,. This chapter describes analytical procedures for the evaluation of elemental impurities that are suitable for the limits described in. already established general assays and tests (e.g., titrimetric method of water determination, bacterial endotoxins test). — during early stages of method development, the robustness of methods should be. — product quality tests for topically applied drug products. method validation ensures a test is reliable and reproducible.
From sepha.com
USP Chapter 1207 CCIT Test Methods Summary Sepha Usp Test Method There are eight (8) different permeation methods. usp reference standards are authentic specimens that have been approved by the usp reference. — the following are usp methods for pharmaceutical drugs: assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation. — during early stages of method development, the robustness. Usp Test Method.
From mycoscience.com
MycoScience USP 81 Turbidimetric Testing For Antimicrobial Medical Usp Test Method — product quality tests for topically applied drug products. summary of usp container performance testing key points. usp reference standards are authentic specimens that have been approved by the usp reference. assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation. the dissolution test in a usp drug. Usp Test Method.
From www.laboratuar.com
USP 232 Standard Test Method for Elemental Impurities, Limits Usp Test Method — this advanced guide will help you properly assess usp testing specifications then best plan your testing strategy. the purpose of this chapter is to provide guidance for validating methods for use as alternatives to the official compendial. the guidelines in usp general chapter <<strong>621</strong>> provide a clearer definition of permissible adjustments during method. pharmacopeial articles. Usp Test Method.
From www.researchgate.net
(PDF) Development and Assessment of a USP Apparatus 3 Dissolution Test Usp Test Method pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to be examined. the guidelines in usp general chapter <<strong>621</strong>> provide a clearer definition of permissible adjustments during method. the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates.. Usp Test Method.
From www.slideserve.com
PPT Compendial Verification PowerPoint Presentation, free download Usp Test Method — expert committee: pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to be examined. the guidelines in usp general chapter <<strong>621</strong>> provide a clearer definition of permissible adjustments during method. usp reference standards are authentic specimens that have been approved by the usp reference. the. Usp Test Method.
From microbe-investigations.com
USP 62 Microbiological Testing of NonSterile Products Usp Test Method summary of usp container performance testing key points. the purpose of this chapter is to provide guidance for validating methods for use as alternatives to the official compendial. What you need to know and how to find proven services. usp offers over 7,000 usp reference standards, highly characterized physical specimens of drug substances, excipients, food ingredients, impurities,.. Usp Test Method.
From www.sgpharma.com
Mefloquine Hydrochloride Tablets USP » SGPharma Usp Test Method This chapter describes analytical procedures for the evaluation of elemental impurities that are suitable for the limits described in. assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation. pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to be examined. . Usp Test Method.
From www.wolterskluwer.com
USP Translating low, medium, and highrisk compounding into Usp Test Method — product quality tests for topically applied drug products. the choice of a method is based on factors such as the nature of the product and the required limit of microorganisms. this chapter describes virology test methods applicable to the development of biological product drugs, such as recombinant. chemical tests and assays. the purpose of. Usp Test Method.
From www.news-medical.net
Standardized tapped density testing for pharmaceutical powders Usp Test Method — the following are usp methods for pharmaceutical drugs: method validation ensures a test is reliable and reproducible. This usp tests a preservative’s effectiveness. usp reference standards are authentic specimens that have been approved by the usp reference. — during early stages of method development, the robustness of methods should be. the dissolution test in. Usp Test Method.
From focus-lab.com
USP 71 Sterility Testing FOCUS Laboratories Usp Test Method the guidelines in usp general chapter <<strong>621</strong>> provide a clearer definition of permissible adjustments during method. read answers to frequently asked questions about usp's microbial enumeration of nonsterile products and more specifically,. Á210ñ monosaccharide analysis (new), 8059 á232ñ elemental impurities—limits, 8065 introduction, routes of. — expert committee: method validation ensures a test is reliable and reproducible.. Usp Test Method.
From microbe-investigations.com
USP 71 Sterility Testing Services Microbe Investigations Usp Test Method What you need to know and how to find proven services. Á210ñ monosaccharide analysis (new), 8059 á232ñ elemental impurities—limits, 8065 introduction, routes of. already established general assays and tests (e.g., titrimetric method of water determination, bacterial endotoxins test). — during early stages of method development, the robustness of methods should be. the guidelines in usp general chapter. Usp Test Method.
From www.youtube.com
Microbiology Testing USP requirements for Sterile and Nonsterile Usp Test Method method validation ensures a test is reliable and reproducible. usp reference standards are authentic specimens that have been approved by the usp reference. pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to be examined. Á210ñ monosaccharide analysis (new), 8059 á232ñ elemental impurities—limits, 8065 introduction, routes of. . Usp Test Method.
From www.researchgate.net
(PDF) Evaluation of the Discriminatory Power of USP Dissolution Method Usp Test Method read answers to frequently asked questions about usp's microbial enumeration of nonsterile products and more specifically,. the guidelines in usp general chapter <<strong>621</strong>> provide a clearer definition of permissible adjustments during method. This chapter describes analytical procedures for the evaluation of elemental impurities that are suitable for the limits described in. usp offers over 7,000 usp reference. Usp Test Method.
From ethidelabs.com
Ethide Laboratories Bioburden Testing Techniques And Examination Usp Test Method the purpose of this chapter is to provide guidance for validating methods for use as alternatives to the official compendial. There are eight (8) different permeation methods. the types of chromatography useful in qualitative and quantitative analysis employed in usp procedures are column,. — the following are usp methods for pharmaceutical drugs: pharmacopeial articles are to. Usp Test Method.
From www.youtube.com
USP CHAPTER 1085, “GUIDELINES ON THE ENDOTOXINS TEST” YouTube Usp Test Method This chapter describes analytical procedures for the evaluation of elemental impurities that are suitable for the limits described in. already established general assays and tests (e.g., titrimetric method of water determination, bacterial endotoxins test). — the following are usp methods for pharmaceutical drugs: — during early stages of method development, the robustness of methods should be. There. Usp Test Method.
From www.youtube.com
Know the Disintegration test apparatus usp tablets capsules YouTube Usp Test Method the choice of a method is based on factors such as the nature of the product and the required limit of microorganisms. the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. — product quality tests for topically applied drug products. read answers to frequently asked. Usp Test Method.
From www.studocu.com
USP 71 Sterility Tests USP 35 Microbiological Tests / 〈 71 Usp Test Method Universal tests (see ich guidance. chemical tests and assays. There are eight (8) different permeation methods. usp reference standards are authentic specimens that have been approved by the usp reference. assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation. read answers to frequently asked questions about usp's microbial. Usp Test Method.
From csanalytical.com
USP 1207 CCI for Combination Products Usp Test Method — during early stages of method development, the robustness of methods should be. usp offers over 7,000 usp reference standards, highly characterized physical specimens of drug substances, excipients, food ingredients, impurities,. This chapter describes analytical procedures for the evaluation of elemental impurities that are suitable for the limits described in. assurance of the quality of pharmaceuticals is. Usp Test Method.
From www.biopharminternational.com
USP Mycoplasma Tests A New Regulation for Mycoplasma Testing Usp Test Method the choice of a method is based on factors such as the nature of the product and the required limit of microorganisms. assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation. — product quality tests for topically applied drug products. — expert committee: the guidelines in usp. Usp Test Method.
From www.researchgate.net
HPLCUV chromatograms using organic impurity test method in USP Usp Test Method this chapter describes virology test methods applicable to the development of biological product drugs, such as recombinant. method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. — the following are usp methods for pharmaceutical drugs: usp offers over 7,000 usp reference standards, highly characterized physical. Usp Test Method.
From www.youtube.com
USP 232 and 233 Understanding Method Requirements and Guidance for Usp Test Method — expert committee: What you need to know and how to find proven services. usp offers over 7,000 usp reference standards, highly characterized physical specimens of drug substances, excipients, food ingredients, impurities,. usp reference standards are authentic specimens that have been approved by the usp reference. — this advanced guide will help you properly assess usp. Usp Test Method.
From ethidelabs.com
Ethide Laboratories What Are USP 61 Microbiological And Bioburden Usp Test Method pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to be examined. the choice of a method is based on factors such as the nature of the product and the required limit of microorganisms. chemical tests and assays. Á210ñ monosaccharide analysis (new), 8059 á232ñ elemental impurities—limits, 8065 introduction,. Usp Test Method.
From www.researchgate.net
USP XXIV testing procedure. Download Scientific Diagram Usp Test Method the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. the purpose of this chapter is to provide guidance for validating methods for use as alternatives to the official compendial. — this advanced guide will help you properly assess usp testing specifications then best plan your testing. Usp Test Method.
From www.slideserve.com
PPT Introduction USP Mission PowerPoint Presentation, free download Usp Test Method Universal tests (see ich guidance. chemical tests and assays. pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to be examined. Á210ñ monosaccharide analysis (new), 8059 á232ñ elemental impurities—limits, 8065 introduction, routes of. summary of usp container performance testing key points. — during early stages of method. Usp Test Method.
From microbe-investigations.com
USP 61 Testing for Microbial Enumeration Microbe Investigations Usp Test Method method validation ensures a test is reliable and reproducible. — product quality tests for topically applied drug products. the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. This chapter describes analytical procedures for the evaluation of elemental impurities that are suitable for the limits described in.. Usp Test Method.
From www.americanpharmaceuticalreview.com
Challenges and Opportunities in Developing UptoDate USPNF Excipient Usp Test Method — expert committee: summary of usp container performance testing key points. the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. usp reference standards are authentic specimens that have been approved by the usp reference. What you need to know and how to find proven services.. Usp Test Method.
From www.slideserve.com
PPT Sterility Testing PowerPoint Presentation, free download ID6352798 Usp Test Method read answers to frequently asked questions about usp's microbial enumeration of nonsterile products and more specifically,. Á210ñ monosaccharide analysis (new), 8059 á232ñ elemental impurities—limits, 8065 introduction, routes of. — during early stages of method development, the robustness of methods should be. method validation ensures a test is reliable and reproducible. — the following are usp methods. Usp Test Method.
From www.mac-mod.com
Paclitaxel Assay (USP Monograph Method) Usp Test Method assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation. the purpose of this chapter is to provide guidance for validating methods for use as alternatives to the official compendial. Universal tests (see ich guidance. — the following are usp methods for pharmaceutical drugs: usp reference standards are authentic. Usp Test Method.
From studylib.net
A novel USP apparatus 4 based release testing method for dispersed systems Usp Test Method pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to be examined. the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust. Usp Test Method.
From pkgcompliance.com
USP 671 Testing Alternative MVTR Test Packaging Compliance Labs Usp Test Method There are eight (8) different permeation methods. the choice of a method is based on factors such as the nature of the product and the required limit of microorganisms. the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. the purpose of this chapter is to provide. Usp Test Method.
From here2grow.com
Analytical Testing Here2Grow Cosmetics & Homecare Labs U.K. Usp Test Method Universal tests (see ich guidance. This usp tests a preservative’s effectiveness. the types of chromatography useful in qualitative and quantitative analysis employed in usp procedures are column,. — expert committee: usp offers over 7,000 usp reference standards, highly characterized physical specimens of drug substances, excipients, food ingredients, impurities,. summary of usp container performance testing key points.. Usp Test Method.
From www.waters.com
Method Modernization for Routine Analysis of Biotherapeutics as Part of Usp Test Method pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to be examined. method validation ensures a test is reliable and reproducible. read answers to frequently asked questions about usp's microbial enumeration of nonsterile products and more specifically,. this chapter describes virology test methods applicable to the development. Usp Test Method.
From www.pharmamanufacturing.com
Pharmaceutical USP Regulations USP Updates and for Microbial Testing Usp Test Method the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. the purpose of this chapter is to provide guidance for validating methods for use as alternatives to the official compendial. — product quality tests for topically applied drug products. method validation of a titration ensures that. Usp Test Method.
From www.researchgate.net
Method Comparison Analysis Data with USP Method (F and Ttest Usp Test Method method validation ensures a test is reliable and reproducible. already established general assays and tests (e.g., titrimetric method of water determination, bacterial endotoxins test). usp offers over 7,000 usp reference standards, highly characterized physical specimens of drug substances, excipients, food ingredients, impurities,. chemical tests and assays. What you need to know and how to find proven. Usp Test Method.
From www.laboratuar.com
USP 711 Standard Test Method for Dissolution Usp Test Method summary of usp container performance testing key points. the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. Á210ñ monosaccharide analysis (new), 8059 á232ñ elemental impurities—limits, 8065 introduction, routes of. — during early stages of method development, the robustness of methods should be. — this advanced. Usp Test Method.