Endpoints Biomarker . A clinical trial’s “endpoints” are measurements of what happens to people in the trial. The biomarkers, endpoints and other tools (best) glossary defines a biomarker as a defined characteristic that is measured as an. Working together with the goals of improving communication, aligning expectations, and improving scientific understanding, the two. A defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an exposure or. A biomarker used to detect or confirm presence of a disease or condition of interest or to identify individuals with a subtype of the disease. The biomarkers, endpoints, and other tools resource is a living document developed jointly by fda and the national institutes of. When a trial is intended to evaluate the efficacy and safety of.
from www.slideshare.net
The biomarkers, endpoints, and other tools resource is a living document developed jointly by fda and the national institutes of. The biomarkers, endpoints and other tools (best) glossary defines a biomarker as a defined characteristic that is measured as an. Working together with the goals of improving communication, aligning expectations, and improving scientific understanding, the two. When a trial is intended to evaluate the efficacy and safety of. A biomarker used to detect or confirm presence of a disease or condition of interest or to identify individuals with a subtype of the disease. A defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an exposure or. A clinical trial’s “endpoints” are measurements of what happens to people in the trial.
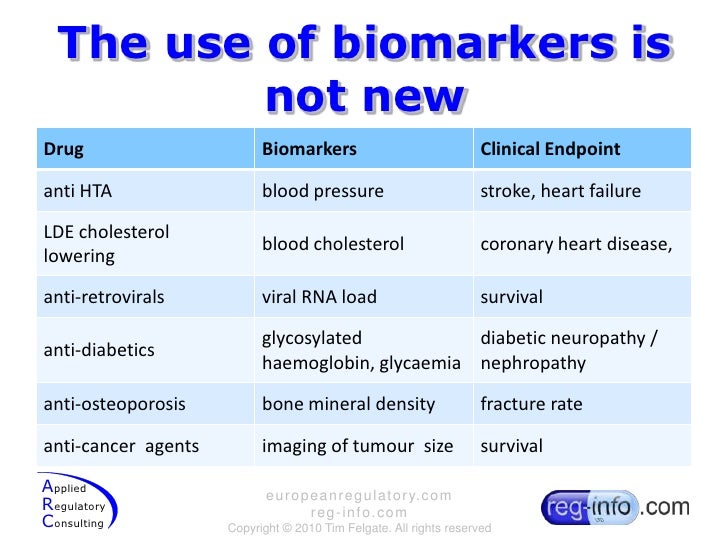
Surrogate endpoints a regulatory review
Endpoints Biomarker The biomarkers, endpoints and other tools (best) glossary defines a biomarker as a defined characteristic that is measured as an. A defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an exposure or. A biomarker used to detect or confirm presence of a disease or condition of interest or to identify individuals with a subtype of the disease. The biomarkers, endpoints and other tools (best) glossary defines a biomarker as a defined characteristic that is measured as an. Working together with the goals of improving communication, aligning expectations, and improving scientific understanding, the two. When a trial is intended to evaluate the efficacy and safety of. The biomarkers, endpoints, and other tools resource is a living document developed jointly by fda and the national institutes of. A clinical trial’s “endpoints” are measurements of what happens to people in the trial.
From www.researchgate.net
Correlation of validated tear biomarkers with clinical endpoints. A Endpoints Biomarker A biomarker used to detect or confirm presence of a disease or condition of interest or to identify individuals with a subtype of the disease. The biomarkers, endpoints and other tools (best) glossary defines a biomarker as a defined characteristic that is measured as an. When a trial is intended to evaluate the efficacy and safety of. Working together with. Endpoints Biomarker.
From www.ncbi.nlm.nih.gov
Presentation by Thomas Fleming Biomarkers and Surrogate Endpoints in Endpoints Biomarker The biomarkers, endpoints, and other tools resource is a living document developed jointly by fda and the national institutes of. Working together with the goals of improving communication, aligning expectations, and improving scientific understanding, the two. A defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an exposure or. The biomarkers,. Endpoints Biomarker.
From slidetodoc.com
Biomarkers and Surrogate Endpoints in Drug Development Technical Endpoints Biomarker The biomarkers, endpoints and other tools (best) glossary defines a biomarker as a defined characteristic that is measured as an. A biomarker used to detect or confirm presence of a disease or condition of interest or to identify individuals with a subtype of the disease. When a trial is intended to evaluate the efficacy and safety of. A clinical trial’s. Endpoints Biomarker.
From www.researchgate.net
A new datadriven approach of biomarker discovery that can utilize Endpoints Biomarker When a trial is intended to evaluate the efficacy and safety of. Working together with the goals of improving communication, aligning expectations, and improving scientific understanding, the two. A clinical trial’s “endpoints” are measurements of what happens to people in the trial. The biomarkers, endpoints and other tools (best) glossary defines a biomarker as a defined characteristic that is measured. Endpoints Biomarker.
From www.blog-nanoentek.com
Why are the biomarkers so important? Endpoints Biomarker A clinical trial’s “endpoints” are measurements of what happens to people in the trial. When a trial is intended to evaluate the efficacy and safety of. The biomarkers, endpoints, and other tools resource is a living document developed jointly by fda and the national institutes of. A defined characteristic that is measured as an indicator of normal biological processes, pathogenic. Endpoints Biomarker.
From www.artfulcompliance.com
00068 Biomarker Types by Disease Progress Endpoints Biomarker The biomarkers, endpoints, and other tools resource is a living document developed jointly by fda and the national institutes of. A defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an exposure or. Working together with the goals of improving communication, aligning expectations, and improving scientific understanding, the two. The biomarkers,. Endpoints Biomarker.
From slideplayer.com
Biomarkers as Endpoints ppt download Endpoints Biomarker A biomarker used to detect or confirm presence of a disease or condition of interest or to identify individuals with a subtype of the disease. When a trial is intended to evaluate the efficacy and safety of. A defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an exposure or. A. Endpoints Biomarker.
From www.slideserve.com
PPT Update on Biomarker and Surrogate Endpoint Qualification Endpoints Biomarker A biomarker used to detect or confirm presence of a disease or condition of interest or to identify individuals with a subtype of the disease. Working together with the goals of improving communication, aligning expectations, and improving scientific understanding, the two. The biomarkers, endpoints and other tools (best) glossary defines a biomarker as a defined characteristic that is measured as. Endpoints Biomarker.
From www.slideserve.com
PPT A Framework for Biomarker and Surrogate Endpoint Use in Drug Endpoints Biomarker A defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an exposure or. Working together with the goals of improving communication, aligning expectations, and improving scientific understanding, the two. The biomarkers, endpoints, and other tools resource is a living document developed jointly by fda and the national institutes of. When a. Endpoints Biomarker.
From myonemedicalsource.com
What is a biomarker? MOMS Endpoints Biomarker A defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an exposure or. The biomarkers, endpoints and other tools (best) glossary defines a biomarker as a defined characteristic that is measured as an. A clinical trial’s “endpoints” are measurements of what happens to people in the trial. A biomarker used to. Endpoints Biomarker.
From www.agappe.com
Biomarkers and its types Endpoints Biomarker When a trial is intended to evaluate the efficacy and safety of. Working together with the goals of improving communication, aligning expectations, and improving scientific understanding, the two. A defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an exposure or. The biomarkers, endpoints and other tools (best) glossary defines a. Endpoints Biomarker.
From www.researchgate.net
Biomarker endpoints after treatment with vehicle (A, D, G and J Endpoints Biomarker Working together with the goals of improving communication, aligning expectations, and improving scientific understanding, the two. The biomarkers, endpoints, and other tools resource is a living document developed jointly by fda and the national institutes of. A defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an exposure or. The biomarkers,. Endpoints Biomarker.
From www.meditrial.net
Clinical trials, how biomarkers help research Meditrial Endpoints Biomarker The biomarkers, endpoints, and other tools resource is a living document developed jointly by fda and the national institutes of. A defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an exposure or. A biomarker used to detect or confirm presence of a disease or condition of interest or to identify. Endpoints Biomarker.
From a2cps.org
Biomarkers Endpoints Biomarker The biomarkers, endpoints, and other tools resource is a living document developed jointly by fda and the national institutes of. The biomarkers, endpoints and other tools (best) glossary defines a biomarker as a defined characteristic that is measured as an. A defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an. Endpoints Biomarker.
From phrma.org
Word of the Month Biomarkers and surrogate endpoints Endpoints Biomarker The biomarkers, endpoints and other tools (best) glossary defines a biomarker as a defined characteristic that is measured as an. A clinical trial’s “endpoints” are measurements of what happens to people in the trial. The biomarkers, endpoints, and other tools resource is a living document developed jointly by fda and the national institutes of. Working together with the goals of. Endpoints Biomarker.
From www.researchgate.net
Changes in intermediate endpoint biomarkers of PCa observed in Phase II Endpoints Biomarker A defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an exposure or. The biomarkers, endpoints, and other tools resource is a living document developed jointly by fda and the national institutes of. A clinical trial’s “endpoints” are measurements of what happens to people in the trial. A biomarker used to. Endpoints Biomarker.
From www.element.com
Biomarkers 101 Intro to Biomarker Analysis Element Element Endpoints Biomarker When a trial is intended to evaluate the efficacy and safety of. A defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an exposure or. A biomarker used to detect or confirm presence of a disease or condition of interest or to identify individuals with a subtype of the disease. Working. Endpoints Biomarker.
From www.slideserve.com
PPT Transition of Biomarkers to Surrogate Endpoints A Critical Path Endpoints Biomarker A biomarker used to detect or confirm presence of a disease or condition of interest or to identify individuals with a subtype of the disease. When a trial is intended to evaluate the efficacy and safety of. Working together with the goals of improving communication, aligning expectations, and improving scientific understanding, the two. The biomarkers, endpoints and other tools (best). Endpoints Biomarker.
From slideplayer.com
A Statistician’s Perspective on Biomarkers in Drug Development ppt Endpoints Biomarker The biomarkers, endpoints, and other tools resource is a living document developed jointly by fda and the national institutes of. When a trial is intended to evaluate the efficacy and safety of. The biomarkers, endpoints and other tools (best) glossary defines a biomarker as a defined characteristic that is measured as an. A clinical trial’s “endpoints” are measurements of what. Endpoints Biomarker.
From imagingendpoints.com
Imaging Biomarkers Imaging Endpoints Endpoints Biomarker The biomarkers, endpoints and other tools (best) glossary defines a biomarker as a defined characteristic that is measured as an. When a trial is intended to evaluate the efficacy and safety of. A biomarker used to detect or confirm presence of a disease or condition of interest or to identify individuals with a subtype of the disease. A clinical trial’s. Endpoints Biomarker.
From www.researchgate.net
Baseline and endpoint biomarker levels according to treatment and Endpoints Biomarker A clinical trial’s “endpoints” are measurements of what happens to people in the trial. The biomarkers, endpoints, and other tools resource is a living document developed jointly by fda and the national institutes of. Working together with the goals of improving communication, aligning expectations, and improving scientific understanding, the two. A defined characteristic that is measured as an indicator of. Endpoints Biomarker.
From www.researchgate.net
Imaging biomarkers. The key characteristic for the detection Endpoints Biomarker A biomarker used to detect or confirm presence of a disease or condition of interest or to identify individuals with a subtype of the disease. Working together with the goals of improving communication, aligning expectations, and improving scientific understanding, the two. The biomarkers, endpoints and other tools (best) glossary defines a biomarker as a defined characteristic that is measured as. Endpoints Biomarker.
From www.researchgate.net
Multivariable analysis of each endpoint including all eight biomarkers Endpoints Biomarker A defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an exposure or. The biomarkers, endpoints and other tools (best) glossary defines a biomarker as a defined characteristic that is measured as an. A clinical trial’s “endpoints” are measurements of what happens to people in the trial. When a trial is. Endpoints Biomarker.
From www.boleary.com
O’Leary’s Blog Regulatory considerations for digital surrogate Endpoints Biomarker The biomarkers, endpoints, and other tools resource is a living document developed jointly by fda and the national institutes of. A biomarker used to detect or confirm presence of a disease or condition of interest or to identify individuals with a subtype of the disease. Working together with the goals of improving communication, aligning expectations, and improving scientific understanding, the. Endpoints Biomarker.
From www.slideshare.net
Surrogate endpoints a regulatory review Endpoints Biomarker The biomarkers, endpoints, and other tools resource is a living document developed jointly by fda and the national institutes of. A clinical trial’s “endpoints” are measurements of what happens to people in the trial. A biomarker used to detect or confirm presence of a disease or condition of interest or to identify individuals with a subtype of the disease. Working. Endpoints Biomarker.
From www.youtube.com
Biomarkers and Surrogate Endpoints in Drug Development YouTube Endpoints Biomarker A clinical trial’s “endpoints” are measurements of what happens to people in the trial. Working together with the goals of improving communication, aligning expectations, and improving scientific understanding, the two. When a trial is intended to evaluate the efficacy and safety of. The biomarkers, endpoints, and other tools resource is a living document developed jointly by fda and the national. Endpoints Biomarker.
From www.youtube.com
Biomarkers & Clinical Endpoints NIH Foundation YouTube Endpoints Biomarker When a trial is intended to evaluate the efficacy and safety of. A clinical trial’s “endpoints” are measurements of what happens to people in the trial. The biomarkers, endpoints and other tools (best) glossary defines a biomarker as a defined characteristic that is measured as an. A biomarker used to detect or confirm presence of a disease or condition of. Endpoints Biomarker.
From www.ncbi.nlm.nih.gov
Figure 2. [Example of a biomarker that...]. BEST (Biomarkers Endpoints Biomarker A defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an exposure or. Working together with the goals of improving communication, aligning expectations, and improving scientific understanding, the two. When a trial is intended to evaluate the efficacy and safety of. A clinical trial’s “endpoints” are measurements of what happens to. Endpoints Biomarker.
From slideplayer.com
Biomarkers as Endpoints ppt download Endpoints Biomarker A defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an exposure or. When a trial is intended to evaluate the efficacy and safety of. A biomarker used to detect or confirm presence of a disease or condition of interest or to identify individuals with a subtype of the disease. Working. Endpoints Biomarker.
From www.researchgate.net
Baseline inflammation biomarkers associated with the primary endpoint Endpoints Biomarker When a trial is intended to evaluate the efficacy and safety of. A defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an exposure or. The biomarkers, endpoints, and other tools resource is a living document developed jointly by fda and the national institutes of. A clinical trial’s “endpoints” are measurements. Endpoints Biomarker.
From www.researchgate.net
Validation of a biomarker (or intermediate endpoint) as a surrogate for Endpoints Biomarker When a trial is intended to evaluate the efficacy and safety of. The biomarkers, endpoints and other tools (best) glossary defines a biomarker as a defined characteristic that is measured as an. The biomarkers, endpoints, and other tools resource is a living document developed jointly by fda and the national institutes of. A clinical trial’s “endpoints” are measurements of what. Endpoints Biomarker.
From www.researchgate.net
Results from the PGS associations with disease endpoints in the Endpoints Biomarker The biomarkers, endpoints and other tools (best) glossary defines a biomarker as a defined characteristic that is measured as an. A biomarker used to detect or confirm presence of a disease or condition of interest or to identify individuals with a subtype of the disease. When a trial is intended to evaluate the efficacy and safety of. A defined characteristic. Endpoints Biomarker.
From www.researchgate.net
Baseline and endpoint biomarker levels according to treatment and Endpoints Biomarker Working together with the goals of improving communication, aligning expectations, and improving scientific understanding, the two. When a trial is intended to evaluate the efficacy and safety of. A biomarker used to detect or confirm presence of a disease or condition of interest or to identify individuals with a subtype of the disease. The biomarkers, endpoints and other tools (best). Endpoints Biomarker.
From www.artfulcompliance.com
00077 Surrogate Endpoint (Response) Biomarker Endpoints Biomarker A defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an exposure or. The biomarkers, endpoints and other tools (best) glossary defines a biomarker as a defined characteristic that is measured as an. The biomarkers, endpoints, and other tools resource is a living document developed jointly by fda and the national. Endpoints Biomarker.
From slidetodoc.com
Biomarkers in Phase II designs in cancer clinical Endpoints Biomarker A clinical trial’s “endpoints” are measurements of what happens to people in the trial. The biomarkers, endpoints and other tools (best) glossary defines a biomarker as a defined characteristic that is measured as an. A defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an exposure or. The biomarkers, endpoints, and. Endpoints Biomarker.