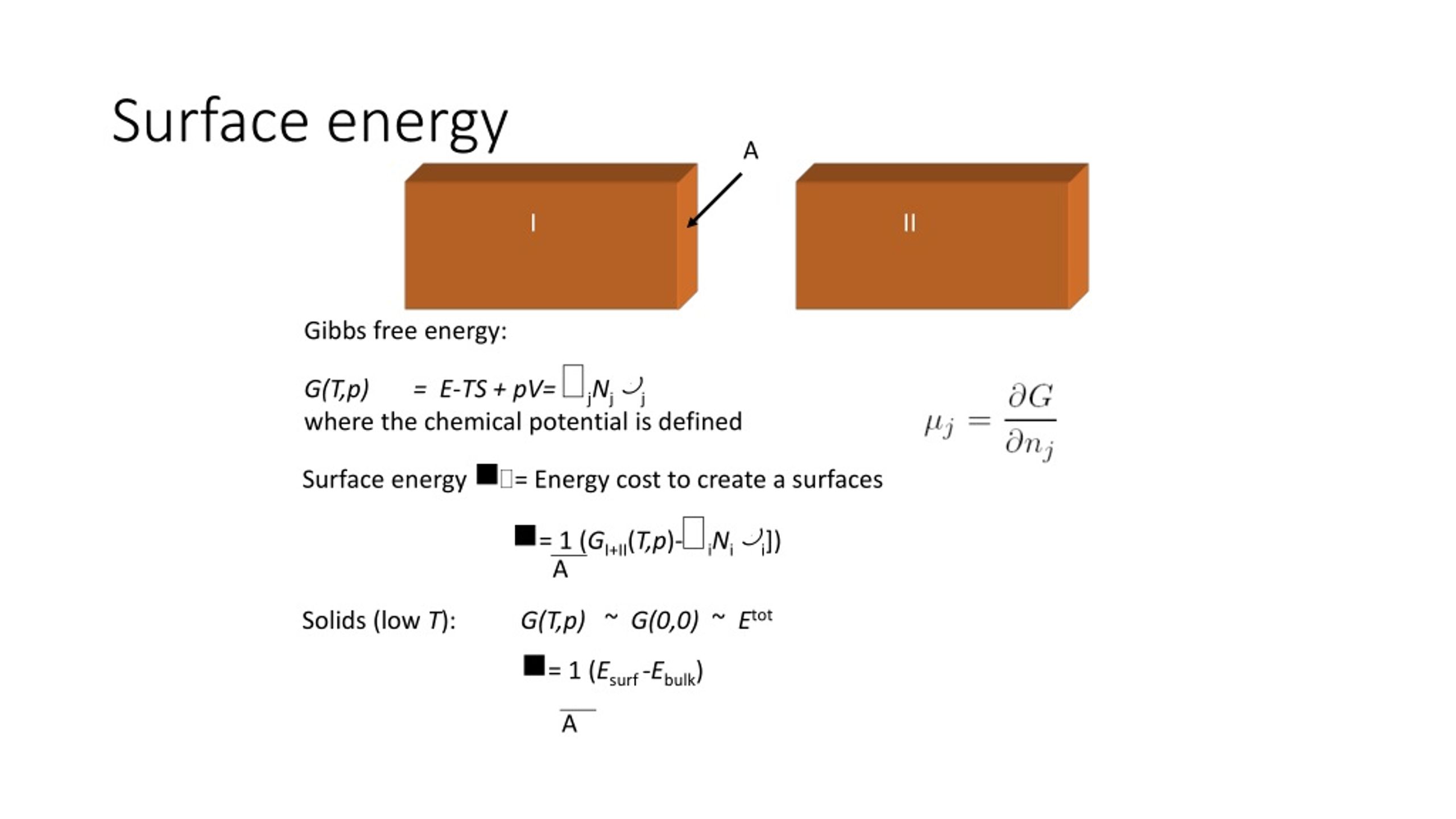
How To Find Surface Energy . surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. In simple language, surface energy can be defined as the work per unit area done by the force that creates the new surface. How much work is needed to increase the surface. it can be used to describe wetting and adhesion between materials, but is not often used quantitatively. surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. a key quantity that is connected with the chemistry of all surfaces is the surface energy. the surface energy of a solid can be determined by measuring several contact angles with a contact angle meter and the sessile drop method. It is also called surface free energy or interfacial free energy. however, from the point of view of thermodynamics, it is easier to think of surface energy.
from www.slideserve.com
the surface energy of a solid can be determined by measuring several contact angles with a contact angle meter and the sessile drop method. It is also called surface free energy or interfacial free energy. surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. however, from the point of view of thermodynamics, it is easier to think of surface energy. it can be used to describe wetting and adhesion between materials, but is not often used quantitatively. In simple language, surface energy can be defined as the work per unit area done by the force that creates the new surface. How much work is needed to increase the surface. surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. a key quantity that is connected with the chemistry of all surfaces is the surface energy.
PPT Surface Chemistry of Materials PowerPoint Presentation, free
How To Find Surface Energy it can be used to describe wetting and adhesion between materials, but is not often used quantitatively. however, from the point of view of thermodynamics, it is easier to think of surface energy. surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. it can be used to describe wetting and adhesion between materials, but is not often used quantitatively. How much work is needed to increase the surface. surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. It is also called surface free energy or interfacial free energy. In simple language, surface energy can be defined as the work per unit area done by the force that creates the new surface. the surface energy of a solid can be determined by measuring several contact angles with a contact angle meter and the sessile drop method. a key quantity that is connected with the chemistry of all surfaces is the surface energy.
From www.researchgate.net
1. Schematic showing the net surface energy balance at the seaair How To Find Surface Energy surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. How much work is needed to increase the surface. surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. In simple language, surface energy can be defined as the work per unit area done by the force that. How To Find Surface Energy.
From www.slideserve.com
PPT Surface energy study PowerPoint Presentation, free download ID How To Find Surface Energy a key quantity that is connected with the chemistry of all surfaces is the surface energy. In simple language, surface energy can be defined as the work per unit area done by the force that creates the new surface. it can be used to describe wetting and adhesion between materials, but is not often used quantitatively. How much. How To Find Surface Energy.
From www.youtube.com
Automated Surface Energy Determination in less than 60 seconds YouTube How To Find Surface Energy it can be used to describe wetting and adhesion between materials, but is not often used quantitatively. How much work is needed to increase the surface. the surface energy of a solid can be determined by measuring several contact angles with a contact angle meter and the sessile drop method. surface energy quantifies the disruption of intermolecular. How To Find Surface Energy.
From www.youtube.com
Solid Surface Energy YouTube How To Find Surface Energy it can be used to describe wetting and adhesion between materials, but is not often used quantitatively. In simple language, surface energy can be defined as the work per unit area done by the force that creates the new surface. however, from the point of view of thermodynamics, it is easier to think of surface energy. surface. How To Find Surface Energy.
From calculator.academy
Surface Energy Calculator Calculator Academy How To Find Surface Energy however, from the point of view of thermodynamics, it is easier to think of surface energy. In simple language, surface energy can be defined as the work per unit area done by the force that creates the new surface. it can be used to describe wetting and adhesion between materials, but is not often used quantitatively. a. How To Find Surface Energy.
From byjus.com
the change in surface energy when a drop of radius R splits up into How To Find Surface Energy it can be used to describe wetting and adhesion between materials, but is not often used quantitatively. the surface energy of a solid can be determined by measuring several contact angles with a contact angle meter and the sessile drop method. surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. How. How To Find Surface Energy.
From www.onlinelabels.com
Surface Energy and Labels The Unscientific Guide How To Find Surface Energy surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. it can be used to describe wetting and adhesion between materials, but is not often used quantitatively. In simple language, surface energy can be defined as the work per unit area done by the force that creates the new surface. How much work. How To Find Surface Energy.
From www.youtube.com
Estimating Solid Surface Energy YouTube How To Find Surface Energy a key quantity that is connected with the chemistry of all surfaces is the surface energy. It is also called surface free energy or interfacial free energy. however, from the point of view of thermodynamics, it is easier to think of surface energy. the surface energy of a solid can be determined by measuring several contact angles. How To Find Surface Energy.
From www.youtube.com
surface energy SI unit and dimensional formula YouTube How To Find Surface Energy the surface energy of a solid can be determined by measuring several contact angles with a contact angle meter and the sessile drop method. a key quantity that is connected with the chemistry of all surfaces is the surface energy. In simple language, surface energy can be defined as the work per unit area done by the force. How To Find Surface Energy.
From studylib.net
Surface Energy W = 2 How To Find Surface Energy the surface energy of a solid can be determined by measuring several contact angles with a contact angle meter and the sessile drop method. however, from the point of view of thermodynamics, it is easier to think of surface energy. How much work is needed to increase the surface. surface energy quantifies the disruption of intermolecular bonds. How To Find Surface Energy.
From www.dataphysics-instruments.com
How to determine the surface energy of solids How To Find Surface Energy the surface energy of a solid can be determined by measuring several contact angles with a contact angle meter and the sessile drop method. It is also called surface free energy or interfacial free energy. surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. How much work is needed to increase the. How To Find Surface Energy.
From www.cadillacplastic.co.uk
Surface Energy and your Choice of Adhesive? Cadillac Plastic How To Find Surface Energy the surface energy of a solid can be determined by measuring several contact angles with a contact angle meter and the sessile drop method. however, from the point of view of thermodynamics, it is easier to think of surface energy. How much work is needed to increase the surface. surface energy quantifies the disruption of intermolecular bonds. How To Find Surface Energy.
From www.researchgate.net
Surface energies for the , 1, 1p and phases calculated by the DFT How To Find Surface Energy It is also called surface free energy or interfacial free energy. surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. In simple language, surface energy can be defined as the work per unit area done by the force that creates the new surface. How much work is needed to increase the surface. . How To Find Surface Energy.
From www.numerade.com
calculate the surface energy for 100, 110 and 111 planes of the simple How To Find Surface Energy the surface energy of a solid can be determined by measuring several contact angles with a contact angle meter and the sessile drop method. It is also called surface free energy or interfacial free energy. surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. How much work is needed to increase the. How To Find Surface Energy.
From www.slideserve.com
PPT DFT Practice Surface Science [based on Chapter 4, Sholl How To Find Surface Energy It is also called surface free energy or interfacial free energy. a key quantity that is connected with the chemistry of all surfaces is the surface energy. surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. How much work is needed to increase the surface. the surface energy of a solid. How To Find Surface Energy.
From www.youtube.com
02 How to calculate surface energy of a material using density How To Find Surface Energy surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. a key quantity that is connected with the chemistry of all surfaces is the surface energy. it can be used to describe wetting and adhesion between materials, but is not often used quantitatively. however, from the point of view of thermodynamics,. How To Find Surface Energy.
From www.youtube.com
Surface Energy Relation Between Surface Tension and Surface Energy How To Find Surface Energy In simple language, surface energy can be defined as the work per unit area done by the force that creates the new surface. a key quantity that is connected with the chemistry of all surfaces is the surface energy. How much work is needed to increase the surface. surface energy quantifies the disruption of intermolecular bonds that occurs. How To Find Surface Energy.
From www.learncram.com
Surface Energy Definition, Formula, Units Surface Tension Learn Cram How To Find Surface Energy How much work is needed to increase the surface. surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. it can be used to describe wetting and adhesion between materials, but is not often used quantitatively. however, from the point of view of thermodynamics, it is easier to think of surface energy.. How To Find Surface Energy.
From www.researchgate.net
A plot of Fowkes equation to determine surface energy Download How To Find Surface Energy the surface energy of a solid can be determined by measuring several contact angles with a contact angle meter and the sessile drop method. It is also called surface free energy or interfacial free energy. however, from the point of view of thermodynamics, it is easier to think of surface energy. How much work is needed to increase. How To Find Surface Energy.
From www.slideserve.com
PPT Chapter 2 Physical Chemistry of Solid Surfaces 固态表面的物理化学 How To Find Surface Energy It is also called surface free energy or interfacial free energy. surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. the surface energy of a solid can be determined by measuring several contact angles with a contact angle meter and the sessile drop method. How much work is needed to increase the. How To Find Surface Energy.
From pubs.acs.org
Study on the Surface Energy of Graphene by Contact Angle Measurements How To Find Surface Energy In simple language, surface energy can be defined as the work per unit area done by the force that creates the new surface. it can be used to describe wetting and adhesion between materials, but is not often used quantitatively. the surface energy of a solid can be determined by measuring several contact angles with a contact angle. How To Find Surface Energy.
From www.chegg.com
Problem 1 Surface energies and Wulff construction How To Find Surface Energy however, from the point of view of thermodynamics, it is easier to think of surface energy. surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. the surface energy of a solid can be determined by measuring several contact angles with a contact angle meter and the sessile drop method. How much. How To Find Surface Energy.
From www.slideserve.com
PPT Physical chemistry of solid surfaces PowerPoint Presentation How To Find Surface Energy It is also called surface free energy or interfacial free energy. surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. How much work is needed to increase the surface. the surface energy of a solid can be determined by measuring several contact angles with a contact angle meter and the sessile drop. How To Find Surface Energy.
From www.youtube.com
6 Relation Between Surface Tension and Surface Energy YouTube How To Find Surface Energy It is also called surface free energy or interfacial free energy. a key quantity that is connected with the chemistry of all surfaces is the surface energy. In simple language, surface energy can be defined as the work per unit area done by the force that creates the new surface. the surface energy of a solid can be. How To Find Surface Energy.
From www.youtube.com
Surface Energy test 2 YouTube How To Find Surface Energy In simple language, surface energy can be defined as the work per unit area done by the force that creates the new surface. How much work is needed to increase the surface. a key quantity that is connected with the chemistry of all surfaces is the surface energy. however, from the point of view of thermodynamics, it is. How To Find Surface Energy.
From www.ossila.com
Surface Energy Formula & Definition Ossila How To Find Surface Energy surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. How much work is needed to increase the surface. it can be used to describe wetting and adhesion between materials, but is not often used quantitatively. surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. . How To Find Surface Energy.
From www.slideserve.com
PPT Surface Chemistry of Materials PowerPoint Presentation, free How To Find Surface Energy In simple language, surface energy can be defined as the work per unit area done by the force that creates the new surface. surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. it can be used to describe wetting and adhesion between materials, but is not often used quantitatively. It is also. How To Find Surface Energy.
From www.slideserve.com
PPT Theoretical Methods for Surface Science part I PowerPoint How To Find Surface Energy however, from the point of view of thermodynamics, it is easier to think of surface energy. the surface energy of a solid can be determined by measuring several contact angles with a contact angle meter and the sessile drop method. surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. How much. How To Find Surface Energy.
From www.chegg.com
Solved 7. (16 Points) (a) In a BCC iron crystal, which plane How To Find Surface Energy a key quantity that is connected with the chemistry of all surfaces is the surface energy. surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. It is also called surface free energy or interfacial free energy. surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created.. How To Find Surface Energy.
From www.geeksforgeeks.org
Surface Energy Definition, Formula, Unit & Numericals How To Find Surface Energy it can be used to describe wetting and adhesion between materials, but is not often used quantitatively. In simple language, surface energy can be defined as the work per unit area done by the force that creates the new surface. It is also called surface free energy or interfacial free energy. the surface energy of a solid can. How To Find Surface Energy.
From mavink.com
Surface Tension Units How To Find Surface Energy surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. however, from the point of view of thermodynamics, it is easier to think of surface energy. it can be used to describe wetting and adhesion between materials, but is not often used quantitatively. surface energy quantifies the disruption of intermolecular bonds. How To Find Surface Energy.
From www.youtube.com
How to calculate surface energy of transition metal nanoparticle YouTube How To Find Surface Energy surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. it can be used to describe wetting and adhesion between materials, but is not often used quantitatively. however, from the point of view of thermodynamics, it is easier to think of surface energy. It is also called surface free energy or interfacial. How To Find Surface Energy.
From www.youtube.com
EMA5001 L0905 Surface energy for FCC 220 plane YouTube How To Find Surface Energy In simple language, surface energy can be defined as the work per unit area done by the force that creates the new surface. the surface energy of a solid can be determined by measuring several contact angles with a contact angle meter and the sessile drop method. it can be used to describe wetting and adhesion between materials,. How To Find Surface Energy.
From www.youtube.com
EMA5001 L0903 Surface energy for FCC 111 plane YouTube How To Find Surface Energy it can be used to describe wetting and adhesion between materials, but is not often used quantitatively. How much work is needed to increase the surface. surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. In simple language, surface energy can be defined as the work per unit area done by the. How To Find Surface Energy.
From www.youtube.com
What is Surface Energy (SE)? YouTube How To Find Surface Energy it can be used to describe wetting and adhesion between materials, but is not often used quantitatively. It is also called surface free energy or interfacial free energy. In simple language, surface energy can be defined as the work per unit area done by the force that creates the new surface. the surface energy of a solid can. How To Find Surface Energy.