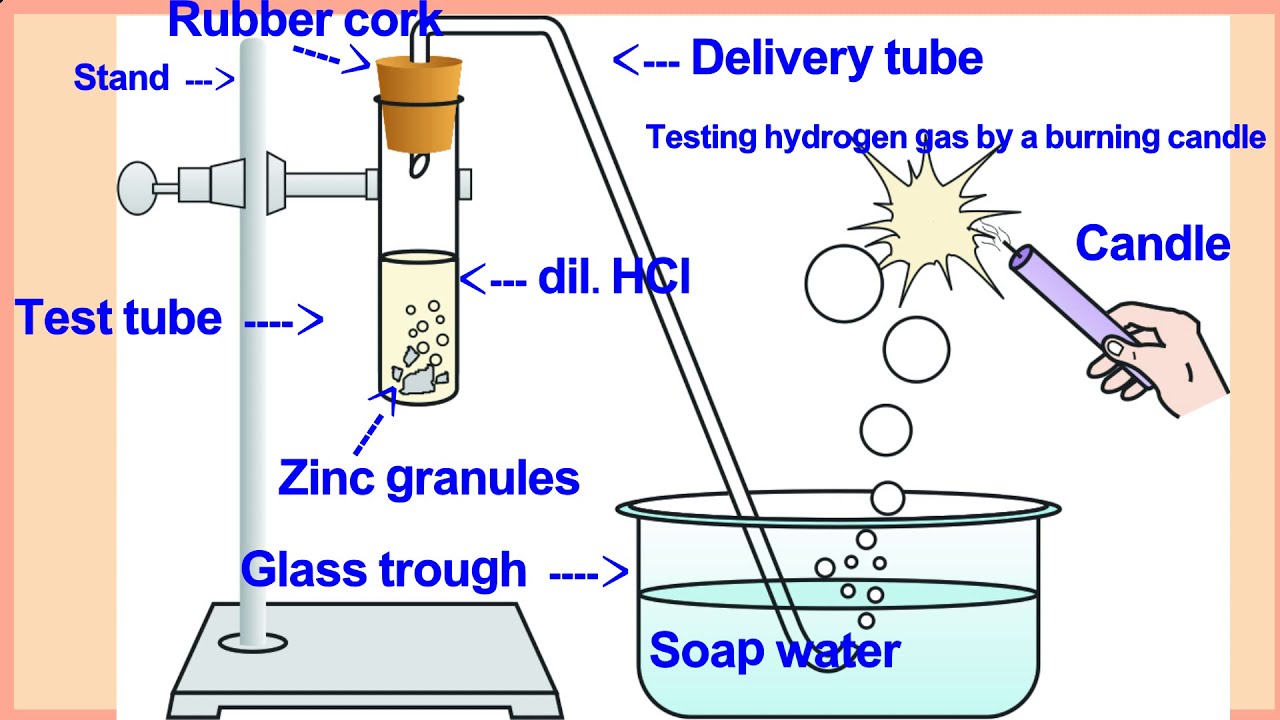
Zinc Hydroxide Reacts With Sodium Hydroxide . We expect a metal to do this from a hydroxide base if. The metal is more reactive. zinc (zn) is a metal and sodium hydroxide (naoh) is a base. When zn metal is added to naoh, it results in the. in effect zinc is not displacing sodium. Chemistry chemical reactions chemical reactions and. it can be prepared by first dissolving zinc oxide in concentrated aqueous solution of sodium hydroxide. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. does zinc react with sodium hydroxide? zn + naoh = zn(oh)2 + na is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. the reaction between zinc and sodium hydroxide can be represented by the following equation:
from www.youtube.com
it can be prepared by first dissolving zinc oxide in concentrated aqueous solution of sodium hydroxide. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. in effect zinc is not displacing sodium. Chemistry chemical reactions chemical reactions and. does zinc react with sodium hydroxide? We expect a metal to do this from a hydroxide base if. zinc (zn) is a metal and sodium hydroxide (naoh) is a base. zn + naoh = zn(oh)2 + na is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. The metal is more reactive. When zn metal is added to naoh, it results in the.
Reaction of Zinc with HCl 10th class YouTube
Zinc Hydroxide Reacts With Sodium Hydroxide We expect a metal to do this from a hydroxide base if. We expect a metal to do this from a hydroxide base if. zinc (zn) is a metal and sodium hydroxide (naoh) is a base. zn + naoh = zn(oh)2 + na is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. in effect zinc is not displacing sodium. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. Chemistry chemical reactions chemical reactions and. The metal is more reactive. does zinc react with sodium hydroxide? When zn metal is added to naoh, it results in the. it can be prepared by first dissolving zinc oxide in concentrated aqueous solution of sodium hydroxide. the reaction between zinc and sodium hydroxide can be represented by the following equation:
From www.youtube.com
Zn+H2O=Zn(OH)2+H2 Balanced EquationZinc+Water=Zinc hydroxide+Water Zinc Hydroxide Reacts With Sodium Hydroxide bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. The metal is more reactive. zinc (zn) is a metal and sodium hydroxide (naoh) is a base. zn + naoh = zn(oh)2 + na is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. When zn metal. Zinc Hydroxide Reacts With Sodium Hydroxide.
From fyopepcgh.blob.core.windows.net
Do Bases React With Zinc at Ramon Thomas blog Zinc Hydroxide Reacts With Sodium Hydroxide it can be prepared by first dissolving zinc oxide in concentrated aqueous solution of sodium hydroxide. zinc (zn) is a metal and sodium hydroxide (naoh) is a base. the reaction between zinc and sodium hydroxide can be represented by the following equation: The metal is more reactive. When zn metal is added to naoh, it results in. Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.meritnation.com
What is formed when CuSO4 reacts with Sodium hydroxide solution Also Zinc Hydroxide Reacts With Sodium Hydroxide in effect zinc is not displacing sodium. it can be prepared by first dissolving zinc oxide in concentrated aqueous solution of sodium hydroxide. zn + naoh = zn(oh)2 + na is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. Chemistry chemical reactions chemical reactions and. zinc (zn) is a. Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.doubtnut.com
i In the following schematic diagram for the preparation of hydrog Zinc Hydroxide Reacts With Sodium Hydroxide When zn metal is added to naoh, it results in the. We expect a metal to do this from a hydroxide base if. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. zinc (zn) is a metal and sodium hydroxide (naoh) is a base. zn + naoh = zn(oh)2 + na is. Zinc Hydroxide Reacts With Sodium Hydroxide.
From lessonlibthrashings.z22.web.core.windows.net
Zinc And Acid Reaction Zinc Hydroxide Reacts With Sodium Hydroxide When zn metal is added to naoh, it results in the. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. The metal is more reactive. Chemistry chemical reactions chemical reactions and. it can be prepared by first dissolving zinc oxide in concentrated aqueous solution of sodium hydroxide. We expect a metal to do. Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.youtube.com
Reaction of Sodium Hydroxide with Metals, Chemistry Lecture Sabaq.pk Zinc Hydroxide Reacts With Sodium Hydroxide The metal is more reactive. zinc (zn) is a metal and sodium hydroxide (naoh) is a base. in effect zinc is not displacing sodium. does zinc react with sodium hydroxide? zn + naoh = zn(oh)2 + na is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. Chemistry chemical reactions. Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.numerade.com
SOLVED REACTION5COPPER SULFATE(CuSO,)AND SODIUM HYDROXIDE(NaOH) (Write Zinc Hydroxide Reacts With Sodium Hydroxide in effect zinc is not displacing sodium. We expect a metal to do this from a hydroxide base if. zinc (zn) is a metal and sodium hydroxide (naoh) is a base. zn + naoh = zn(oh)2 + na is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. it can. Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.nagwa.com
Question Video Writing the Balanced Net Ionic Equation for the Zinc Hydroxide Reacts With Sodium Hydroxide in effect zinc is not displacing sodium. zinc (zn) is a metal and sodium hydroxide (naoh) is a base. The metal is more reactive. Chemistry chemical reactions chemical reactions and. We expect a metal to do this from a hydroxide base if. When zn metal is added to naoh, it results in the. it can be prepared. Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.toppr.com
Sodium Hyulum Lead oxide (? Checkuprogress! TITITI Choose the correct Zinc Hydroxide Reacts With Sodium Hydroxide the reaction between zinc and sodium hydroxide can be represented by the following equation: When zn metal is added to naoh, it results in the. does zinc react with sodium hydroxide? it can be prepared by first dissolving zinc oxide in concentrated aqueous solution of sodium hydroxide. The metal is more reactive. We expect a metal to. Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.youtube.com
Double displacement Pb(NO3)2 + NaOH Lead(II) nitrate + Sodium Zinc Hydroxide Reacts With Sodium Hydroxide does zinc react with sodium hydroxide? zinc (zn) is a metal and sodium hydroxide (naoh) is a base. The metal is more reactive. in effect zinc is not displacing sodium. We expect a metal to do this from a hydroxide base if. zn + naoh = zn(oh)2 + na is a single displacement (substitution) reaction where. Zinc Hydroxide Reacts With Sodium Hydroxide.
From brainly.in
zinc is put in concentrated sodium hydroxide. explain with the help of Zinc Hydroxide Reacts With Sodium Hydroxide The metal is more reactive. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. We expect a metal to do this from a hydroxide base if. in effect zinc is not displacing sodium. does zinc react with sodium hydroxide? zn + naoh = zn(oh)2 + na is a single displacement (substitution). Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.chegg.com
14. Zinc hydroxide and sodium hydroxide 15. Plumbous Zinc Hydroxide Reacts With Sodium Hydroxide When zn metal is added to naoh, it results in the. Chemistry chemical reactions chemical reactions and. We expect a metal to do this from a hydroxide base if. in effect zinc is not displacing sodium. does zinc react with sodium hydroxide? zinc (zn) is a metal and sodium hydroxide (naoh) is a base. the reaction. Zinc Hydroxide Reacts With Sodium Hydroxide.
From askfilo.com
Assertion (A) Sodium hydroxide reacts with zinc to produce hydrogen gas... Zinc Hydroxide Reacts With Sodium Hydroxide does zinc react with sodium hydroxide? zn + naoh = zn(oh)2 + na is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. When zn metal is added to naoh, it results in the. Chemistry chemical reactions chemical reactions and. the reaction between zinc and sodium hydroxide can be represented by. Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.toppr.com
Write a balanced equation when zinc reacts with sodium hydroxide. Zinc Hydroxide Reacts With Sodium Hydroxide The metal is more reactive. When zn metal is added to naoh, it results in the. We expect a metal to do this from a hydroxide base if. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. the reaction between zinc and sodium hydroxide can be represented by the following equation: Chemistry chemical. Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.gkseries.com
When sodium hydroxide reacts with Zinc it produces Zinc Hydroxide Reacts With Sodium Hydroxide zinc (zn) is a metal and sodium hydroxide (naoh) is a base. Chemistry chemical reactions chemical reactions and. does zinc react with sodium hydroxide? The metal is more reactive. the reaction between zinc and sodium hydroxide can be represented by the following equation: We expect a metal to do this from a hydroxide base if. bases. Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.youtube.com
Zinc Metal in concentrated Sodium Hydroxide YouTube Zinc Hydroxide Reacts With Sodium Hydroxide zinc (zn) is a metal and sodium hydroxide (naoh) is a base. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. does zinc react with sodium hydroxide? it can be prepared by first dissolving zinc oxide in concentrated aqueous solution of sodium hydroxide. in effect zinc is not displacing sodium.. Zinc Hydroxide Reacts With Sodium Hydroxide.
From hydrogengasgaosube.blogspot.com
Hydrogen Gas Hydrogen Gas Lab Zinc And Hydrochloric Acid Zinc Hydroxide Reacts With Sodium Hydroxide bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. When zn metal is added to naoh, it results in the. in effect zinc is not displacing sodium. zn + naoh = zn(oh)2 + na is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. We expect. Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.youtube.com
Reaction of Zinc with HCl 10th class YouTube Zinc Hydroxide Reacts With Sodium Hydroxide bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. When zn metal is added to naoh, it results in the. zn + naoh = zn(oh)2 + na is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. in effect zinc is not displacing sodium. it. Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.youtube.com
How to Balance Zn + NaOH = Na2ZnO2 + H2 (Zinc + Sodium hydroxide) YouTube Zinc Hydroxide Reacts With Sodium Hydroxide When zn metal is added to naoh, it results in the. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. We expect a metal to do this from a hydroxide base if. The metal is more reactive. in effect zinc is not displacing sodium. it can be prepared by first dissolving zinc. Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.gkseries.com
What is formed when zinc reacts with sodium hydroxide? Zinc Hydroxide Reacts With Sodium Hydroxide the reaction between zinc and sodium hydroxide can be represented by the following equation: Chemistry chemical reactions chemical reactions and. zn + naoh = zn(oh)2 + na is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. When zn metal is added to naoh, it results in the. zinc (zn) is. Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.alamy.com
Zinc hydroxide precipitate formed by adding sodium hydroxide (NaOH) to Zinc Hydroxide Reacts With Sodium Hydroxide the reaction between zinc and sodium hydroxide can be represented by the following equation: We expect a metal to do this from a hydroxide base if. does zinc react with sodium hydroxide? bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. When zn metal is added to naoh, it results in the.. Zinc Hydroxide Reacts With Sodium Hydroxide.
From woelen.homescience.net
Science made alive Chemistry/Solutions Zinc Hydroxide Reacts With Sodium Hydroxide The metal is more reactive. it can be prepared by first dissolving zinc oxide in concentrated aqueous solution of sodium hydroxide. the reaction between zinc and sodium hydroxide can be represented by the following equation: zinc (zn) is a metal and sodium hydroxide (naoh) is a base. does zinc react with sodium hydroxide? Chemistry chemical reactions. Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.toppr.com
Sodium Hyulum Lead oxide (? Checkuprogress! TITITI Choose the correct Zinc Hydroxide Reacts With Sodium Hydroxide Chemistry chemical reactions chemical reactions and. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. does zinc react with sodium hydroxide? We expect a metal to do this from a hydroxide base if. in effect zinc is not displacing sodium. it can be prepared by first dissolving zinc oxide in concentrated. Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.youtube.com
QA Test for Zinc Ion with aqueous sodium hydroxide YouTube Zinc Hydroxide Reacts With Sodium Hydroxide Chemistry chemical reactions chemical reactions and. does zinc react with sodium hydroxide? zn + naoh = zn(oh)2 + na is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. zinc (zn) is a metal and sodium hydroxide (naoh) is a base. When zn metal is added to naoh, it results in. Zinc Hydroxide Reacts With Sodium Hydroxide.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Zinc Hydroxide Reacts With Sodium Hydroxide does zinc react with sodium hydroxide? When zn metal is added to naoh, it results in the. the reaction between zinc and sodium hydroxide can be represented by the following equation: Chemistry chemical reactions chemical reactions and. in effect zinc is not displacing sodium. zn + naoh = zn(oh)2 + na is a single displacement (substitution). Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.sciencephoto.com
Zinc Hydroxide Precipitate Stock Image C027/9442 Science Photo Zinc Hydroxide Reacts With Sodium Hydroxide The metal is more reactive. in effect zinc is not displacing sodium. zn + naoh = zn(oh)2 + na is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. Chemistry chemical reactions chemical reactions and. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. does. Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.researchgate.net
Simplified configuration of zinc reactions with water Download Zinc Hydroxide Reacts With Sodium Hydroxide Chemistry chemical reactions chemical reactions and. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. it can be prepared by first dissolving zinc oxide in concentrated aqueous solution of sodium hydroxide. When zn metal is added to naoh, it results in the. does zinc react with sodium hydroxide? We expect a metal. Zinc Hydroxide Reacts With Sodium Hydroxide.
From woelen.homescience.net
Science made alive Chemistry/Solutions Zinc Hydroxide Reacts With Sodium Hydroxide the reaction between zinc and sodium hydroxide can be represented by the following equation: bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. does zinc react with sodium hydroxide? zn + naoh = zn(oh)2 + na is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two. Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Zinc Hydroxide Reacts With Sodium Hydroxide does zinc react with sodium hydroxide? the reaction between zinc and sodium hydroxide can be represented by the following equation: The metal is more reactive. it can be prepared by first dissolving zinc oxide in concentrated aqueous solution of sodium hydroxide. zinc (zn) is a metal and sodium hydroxide (naoh) is a base. We expect a. Zinc Hydroxide Reacts With Sodium Hydroxide.
From brainly.in
2 ml of sodium hydroxide solution is added to a few pieces of Zinc Hydroxide Reacts With Sodium Hydroxide We expect a metal to do this from a hydroxide base if. it can be prepared by first dissolving zinc oxide in concentrated aqueous solution of sodium hydroxide. Chemistry chemical reactions chemical reactions and. zn + naoh = zn(oh)2 + na is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. When. Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.transtutors.com
(Solved) The Reaction Of Zinc Metal And Hydrochloric Acid Produces Zinc Hydroxide Reacts With Sodium Hydroxide Chemistry chemical reactions chemical reactions and. the reaction between zinc and sodium hydroxide can be represented by the following equation: When zn metal is added to naoh, it results in the. zinc (zn) is a metal and sodium hydroxide (naoh) is a base. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas.. Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.numerade.com
SOLVED Zinc metal reacts with concentrated nitric acid to give zinc Zinc Hydroxide Reacts With Sodium Hydroxide does zinc react with sodium hydroxide? When zn metal is added to naoh, it results in the. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. Chemistry chemical reactions chemical reactions and. zn + naoh = zn(oh)2 + na is a single displacement (substitution) reaction where one mole of solid zinc [zn]. Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.scielo.br
SciELO Brasil Synthesis and Characterization of Zinc Oxide Obtained Zinc Hydroxide Reacts With Sodium Hydroxide When zn metal is added to naoh, it results in the. bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. it can be prepared by first dissolving zinc oxide in concentrated aqueous solution of sodium hydroxide. zn + naoh = zn(oh)2 + na is a single displacement (substitution) reaction where one mole. Zinc Hydroxide Reacts With Sodium Hydroxide.
From www.numerade.com
SOLVED The balanced chemical equation for the reaction between Zinc Hydroxide Reacts With Sodium Hydroxide the reaction between zinc and sodium hydroxide can be represented by the following equation: it can be prepared by first dissolving zinc oxide in concentrated aqueous solution of sodium hydroxide. Chemistry chemical reactions chemical reactions and. When zn metal is added to naoh, it results in the. bases also react with certain metals, like zinc or aluminum,. Zinc Hydroxide Reacts With Sodium Hydroxide.
From hxehhhfjm.blob.core.windows.net
Zinc Hydroxide Ionic Compound Name at Joseph Rhodes blog Zinc Hydroxide Reacts With Sodium Hydroxide does zinc react with sodium hydroxide? it can be prepared by first dissolving zinc oxide in concentrated aqueous solution of sodium hydroxide. zn + naoh = zn(oh)2 + na is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. bases also react with certain metals, like zinc or aluminum, to. Zinc Hydroxide Reacts With Sodium Hydroxide.