Iv Labeling Requirements . Find out the best practices, protocols and labels for multiple iv lines, hazardous. See the national standardisation, minimum. An analysis of the medication use process guided our development of the “ideal”. Learn how to label iv line tubing properly to reduce adverse drug events and improve patient safety. This document provides guidance for safe preparation of compounded sterile preparations (csps), including iv. Absence of a uniform pattern of structuring the labels in. The results found on labeling iv therapy devices in the icu indicated: Asa supports the safe practice of spiking commercially available intravenous (iv) fluid bags within 24 hours without risk of. Learn how to label injectable medicines, fluids and delivery devices to prevent medicine administration errors and improve patient safety.
from www.drugwatch.com
An analysis of the medication use process guided our development of the “ideal”. Asa supports the safe practice of spiking commercially available intravenous (iv) fluid bags within 24 hours without risk of. Learn how to label injectable medicines, fluids and delivery devices to prevent medicine administration errors and improve patient safety. This document provides guidance for safe preparation of compounded sterile preparations (csps), including iv. Learn how to label iv line tubing properly to reduce adverse drug events and improve patient safety. The results found on labeling iv therapy devices in the icu indicated: Absence of a uniform pattern of structuring the labels in. Find out the best practices, protocols and labels for multiple iv lines, hazardous. See the national standardisation, minimum.
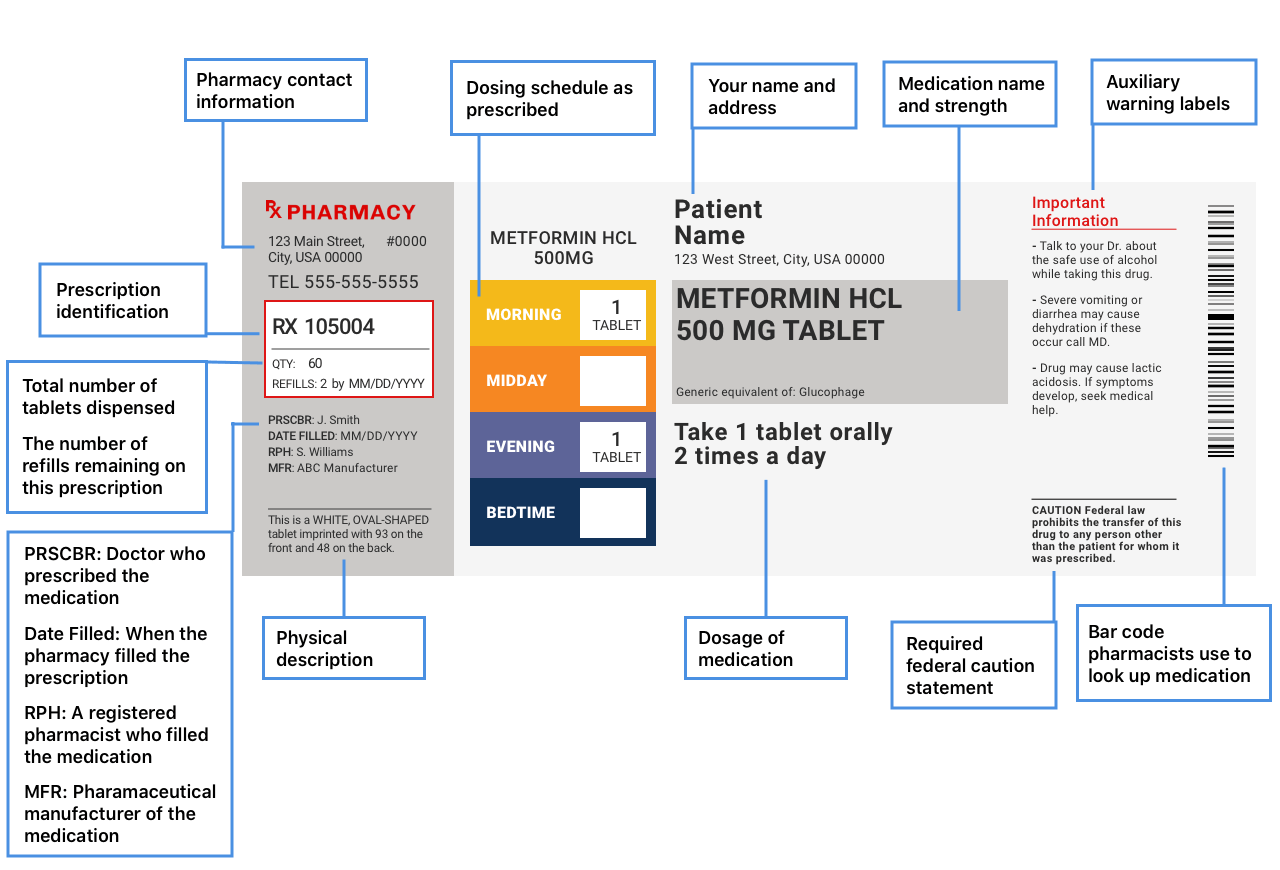
How to Read OvertheCounter and Prescription Drug Labels
Iv Labeling Requirements Learn how to label iv line tubing properly to reduce adverse drug events and improve patient safety. An analysis of the medication use process guided our development of the “ideal”. Find out the best practices, protocols and labels for multiple iv lines, hazardous. Asa supports the safe practice of spiking commercially available intravenous (iv) fluid bags within 24 hours without risk of. See the national standardisation, minimum. The results found on labeling iv therapy devices in the icu indicated: Absence of a uniform pattern of structuring the labels in. This document provides guidance for safe preparation of compounded sterile preparations (csps), including iv. Learn how to label iv line tubing properly to reduce adverse drug events and improve patient safety. Learn how to label injectable medicines, fluids and delivery devices to prevent medicine administration errors and improve patient safety.
From www.unitedadlabel.com
Pt. Name Room, IV Medication Added Label, 21/2" x 21/2" United Ad Label Iv Labeling Requirements See the national standardisation, minimum. An analysis of the medication use process guided our development of the “ideal”. Absence of a uniform pattern of structuring the labels in. Find out the best practices, protocols and labels for multiple iv lines, hazardous. Learn how to label iv line tubing properly to reduce adverse drug events and improve patient safety. This document. Iv Labeling Requirements.
From nursing-procedures.blogspot.com
Nursing Procedures Administering Medications by IV Piggyback to an Iv Labeling Requirements See the national standardisation, minimum. The results found on labeling iv therapy devices in the icu indicated: Asa supports the safe practice of spiking commercially available intravenous (iv) fluid bags within 24 hours without risk of. An analysis of the medication use process guided our development of the “ideal”. Learn how to label iv line tubing properly to reduce adverse. Iv Labeling Requirements.
From www.mermed.com.au
IntraVENOUS label Mermed Medical Supplies Iv Labeling Requirements The results found on labeling iv therapy devices in the icu indicated: Absence of a uniform pattern of structuring the labels in. This document provides guidance for safe preparation of compounded sterile preparations (csps), including iv. Learn how to label iv line tubing properly to reduce adverse drug events and improve patient safety. See the national standardisation, minimum. Asa supports. Iv Labeling Requirements.
From www.pinterest.fr
IV Fluids and Solutions Quick Reference Guide for Nurses and medical Iv Labeling Requirements This document provides guidance for safe preparation of compounded sterile preparations (csps), including iv. See the national standardisation, minimum. An analysis of the medication use process guided our development of the “ideal”. The results found on labeling iv therapy devices in the icu indicated: Learn how to label injectable medicines, fluids and delivery devices to prevent medicine administration errors and. Iv Labeling Requirements.
From hub.arkansasbluecross.com
Deciphering Your Prescription Medication Label Blueprint Iv Labeling Requirements Asa supports the safe practice of spiking commercially available intravenous (iv) fluid bags within 24 hours without risk of. This document provides guidance for safe preparation of compounded sterile preparations (csps), including iv. The results found on labeling iv therapy devices in the icu indicated: See the national standardisation, minimum. Learn how to label injectable medicines, fluids and delivery devices. Iv Labeling Requirements.
From europepmc.org
Improving the usability of intravenous medication labels to support Iv Labeling Requirements Learn how to label injectable medicines, fluids and delivery devices to prevent medicine administration errors and improve patient safety. Learn how to label iv line tubing properly to reduce adverse drug events and improve patient safety. An analysis of the medication use process guided our development of the “ideal”. Find out the best practices, protocols and labels for multiple iv. Iv Labeling Requirements.
From defries.com.au
Label Drug Cephazolin Defries Industries Hospitals Trust Defries Iv Labeling Requirements The results found on labeling iv therapy devices in the icu indicated: See the national standardisation, minimum. Absence of a uniform pattern of structuring the labels in. This document provides guidance for safe preparation of compounded sterile preparations (csps), including iv. Find out the best practices, protocols and labels for multiple iv lines, hazardous. Learn how to label injectable medicines,. Iv Labeling Requirements.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Iv Labeling Requirements Asa supports the safe practice of spiking commercially available intravenous (iv) fluid bags within 24 hours without risk of. This document provides guidance for safe preparation of compounded sterile preparations (csps), including iv. Find out the best practices, protocols and labels for multiple iv lines, hazardous. Learn how to label iv line tubing properly to reduce adverse drug events and. Iv Labeling Requirements.
From shamrocklabels.com
IV Medication Labels IV Tubing Labels Iv Labeling Requirements Asa supports the safe practice of spiking commercially available intravenous (iv) fluid bags within 24 hours without risk of. Learn how to label iv line tubing properly to reduce adverse drug events and improve patient safety. The results found on labeling iv therapy devices in the icu indicated: This document provides guidance for safe preparation of compounded sterile preparations (csps),. Iv Labeling Requirements.
From www.pinterest.com
Free Download Intravenous (IV) Fluids and Solutions Quick Reference Iv Labeling Requirements Learn how to label injectable medicines, fluids and delivery devices to prevent medicine administration errors and improve patient safety. This document provides guidance for safe preparation of compounded sterile preparations (csps), including iv. Absence of a uniform pattern of structuring the labels in. See the national standardisation, minimum. An analysis of the medication use process guided our development of the. Iv Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Iv Labeling Requirements An analysis of the medication use process guided our development of the “ideal”. Learn how to label injectable medicines, fluids and delivery devices to prevent medicine administration errors and improve patient safety. Asa supports the safe practice of spiking commercially available intravenous (iv) fluid bags within 24 hours without risk of. See the national standardisation, minimum. This document provides guidance. Iv Labeling Requirements.
From www.pinterest.com
50+ IV Therapy Tips and Tricks The Ultimate Guide Iv therapy Iv Labeling Requirements An analysis of the medication use process guided our development of the “ideal”. Learn how to label injectable medicines, fluids and delivery devices to prevent medicine administration errors and improve patient safety. Learn how to label iv line tubing properly to reduce adverse drug events and improve patient safety. The results found on labeling iv therapy devices in the icu. Iv Labeling Requirements.
From www.waltersmedical.co.uk
Intravenous additive label 60 x 80mm Walters Medical Iv Labeling Requirements See the national standardisation, minimum. Find out the best practices, protocols and labels for multiple iv lines, hazardous. Absence of a uniform pattern of structuring the labels in. The results found on labeling iv therapy devices in the icu indicated: Learn how to label injectable medicines, fluids and delivery devices to prevent medicine administration errors and improve patient safety. This. Iv Labeling Requirements.
From www.graylinemedical.com
Briggs Healthcare IV Labels "Intravenous Solution Additives" Label Iv Labeling Requirements See the national standardisation, minimum. The results found on labeling iv therapy devices in the icu indicated: An analysis of the medication use process guided our development of the “ideal”. Learn how to label injectable medicines, fluids and delivery devices to prevent medicine administration errors and improve patient safety. Absence of a uniform pattern of structuring the labels in. Learn. Iv Labeling Requirements.
From www.caresfield.com
3 x 1 Printed "IV Tubing Changed" label, Orange, 885 per roll, 6 rolls Iv Labeling Requirements Find out the best practices, protocols and labels for multiple iv lines, hazardous. Learn how to label injectable medicines, fluids and delivery devices to prevent medicine administration errors and improve patient safety. See the national standardisation, minimum. An analysis of the medication use process guided our development of the “ideal”. The results found on labeling iv therapy devices in the. Iv Labeling Requirements.
From www.unitedadlabel.com
Fluorescent Orange IV Medication Added Label, 3" x 2" United Ad Label Iv Labeling Requirements This document provides guidance for safe preparation of compounded sterile preparations (csps), including iv. An analysis of the medication use process guided our development of the “ideal”. Absence of a uniform pattern of structuring the labels in. Learn how to label injectable medicines, fluids and delivery devices to prevent medicine administration errors and improve patient safety. Asa supports the safe. Iv Labeling Requirements.
From www.fda.gov.ph
Draft for Comments Guidelines on Labeling Requirements of Drug Iv Labeling Requirements Find out the best practices, protocols and labels for multiple iv lines, hazardous. This document provides guidance for safe preparation of compounded sterile preparations (csps), including iv. Absence of a uniform pattern of structuring the labels in. An analysis of the medication use process guided our development of the “ideal”. Asa supports the safe practice of spiking commercially available intravenous. Iv Labeling Requirements.
From bushcrafttips.com
The Basics of IV Fluids for Emergency Scenarios Iv Labeling Requirements The results found on labeling iv therapy devices in the icu indicated: Learn how to label injectable medicines, fluids and delivery devices to prevent medicine administration errors and improve patient safety. See the national standardisation, minimum. This document provides guidance for safe preparation of compounded sterile preparations (csps), including iv. Absence of a uniform pattern of structuring the labels in.. Iv Labeling Requirements.
From slideserve.com
PPT USP Standards Pharmaceutical Compounding Sterile Preparations Iv Labeling Requirements This document provides guidance for safe preparation of compounded sterile preparations (csps), including iv. Learn how to label injectable medicines, fluids and delivery devices to prevent medicine administration errors and improve patient safety. See the national standardisation, minimum. Find out the best practices, protocols and labels for multiple iv lines, hazardous. Asa supports the safe practice of spiking commercially available. Iv Labeling Requirements.
From europepmc.org
Improving the usability of intravenous medication labels to support Iv Labeling Requirements The results found on labeling iv therapy devices in the icu indicated: See the national standardisation, minimum. Absence of a uniform pattern of structuring the labels in. Learn how to label injectable medicines, fluids and delivery devices to prevent medicine administration errors and improve patient safety. Learn how to label iv line tubing properly to reduce adverse drug events and. Iv Labeling Requirements.
From mermed.com.au
Anaesthetic,IV,Barcode,Surgical,Sterilizing Labels Australia Mermed Iv Labeling Requirements This document provides guidance for safe preparation of compounded sterile preparations (csps), including iv. Find out the best practices, protocols and labels for multiple iv lines, hazardous. Asa supports the safe practice of spiking commercially available intravenous (iv) fluid bags within 24 hours without risk of. Learn how to label iv line tubing properly to reduce adverse drug events and. Iv Labeling Requirements.
From www.bradyid.com
Arc Flash Labeling Requirements Comply with 2024 NFPA 70E Iv Labeling Requirements Absence of a uniform pattern of structuring the labels in. The results found on labeling iv therapy devices in the icu indicated: This document provides guidance for safe preparation of compounded sterile preparations (csps), including iv. See the national standardisation, minimum. Find out the best practices, protocols and labels for multiple iv lines, hazardous. An analysis of the medication use. Iv Labeling Requirements.
From www.youtube.com
How to read a medication label YouTube Iv Labeling Requirements An analysis of the medication use process guided our development of the “ideal”. Absence of a uniform pattern of structuring the labels in. This document provides guidance for safe preparation of compounded sterile preparations (csps), including iv. Find out the best practices, protocols and labels for multiple iv lines, hazardous. See the national standardisation, minimum. Learn how to label iv. Iv Labeling Requirements.
From www.slideserve.com
PPT Parenteral Therapy PowerPoint Presentation, free download ID Iv Labeling Requirements Asa supports the safe practice of spiking commercially available intravenous (iv) fluid bags within 24 hours without risk of. This document provides guidance for safe preparation of compounded sterile preparations (csps), including iv. Learn how to label injectable medicines, fluids and delivery devices to prevent medicine administration errors and improve patient safety. The results found on labeling iv therapy devices. Iv Labeling Requirements.
From ecampusontario.pressbooks.pub
8.4 Priming IV Tubing and Changing IV Fluids and Tubing Clinical Iv Labeling Requirements An analysis of the medication use process guided our development of the “ideal”. Asa supports the safe practice of spiking commercially available intravenous (iv) fluid bags within 24 hours without risk of. The results found on labeling iv therapy devices in the icu indicated: Find out the best practices, protocols and labels for multiple iv lines, hazardous. Absence of a. Iv Labeling Requirements.
From www.sssaustralia.com.au
For Intravenous Use Only Labels 60mm x 50mm Blue SSS Australia SSS Iv Labeling Requirements An analysis of the medication use process guided our development of the “ideal”. The results found on labeling iv therapy devices in the icu indicated: Learn how to label iv line tubing properly to reduce adverse drug events and improve patient safety. See the national standardisation, minimum. This document provides guidance for safe preparation of compounded sterile preparations (csps), including. Iv Labeling Requirements.
From dl-uk.apowersoft.com
Iv Bag Label Template Iv Labeling Requirements This document provides guidance for safe preparation of compounded sterile preparations (csps), including iv. Learn how to label iv line tubing properly to reduce adverse drug events and improve patient safety. Absence of a uniform pattern of structuring the labels in. An analysis of the medication use process guided our development of the “ideal”. See the national standardisation, minimum. Learn. Iv Labeling Requirements.
From www.etsy.com
IV Tubing and Dressing Change Cheat Sheet Etsy Iv Labeling Requirements The results found on labeling iv therapy devices in the icu indicated: See the national standardisation, minimum. Find out the best practices, protocols and labels for multiple iv lines, hazardous. Absence of a uniform pattern of structuring the labels in. Asa supports the safe practice of spiking commercially available intravenous (iv) fluid bags within 24 hours without risk of. This. Iv Labeling Requirements.
From www.unitedadlabel.com
IV System Preprinted Pinfeed Label, 3" x 115/16" United Ad Label Iv Labeling Requirements Learn how to label injectable medicines, fluids and delivery devices to prevent medicine administration errors and improve patient safety. See the national standardisation, minimum. Learn how to label iv line tubing properly to reduce adverse drug events and improve patient safety. Find out the best practices, protocols and labels for multiple iv lines, hazardous. Asa supports the safe practice of. Iv Labeling Requirements.
From www.sssaustralia.com.au
I.V Additive Label (Orange) SSS Australia SSS Australia Medical Iv Labeling Requirements An analysis of the medication use process guided our development of the “ideal”. Absence of a uniform pattern of structuring the labels in. This document provides guidance for safe preparation of compounded sterile preparations (csps), including iv. See the national standardisation, minimum. Learn how to label iv line tubing properly to reduce adverse drug events and improve patient safety. The. Iv Labeling Requirements.
From opentextbc.ca
7.6 Administering Intermittent Intravenous Medication (Secondary Iv Labeling Requirements The results found on labeling iv therapy devices in the icu indicated: This document provides guidance for safe preparation of compounded sterile preparations (csps), including iv. Learn how to label injectable medicines, fluids and delivery devices to prevent medicine administration errors and improve patient safety. Find out the best practices, protocols and labels for multiple iv lines, hazardous. An analysis. Iv Labeling Requirements.
From www.gregoryschmidt.ca
Order Entry UX General Requirements, for Medications — Gregory Schmidt Iv Labeling Requirements Find out the best practices, protocols and labels for multiple iv lines, hazardous. This document provides guidance for safe preparation of compounded sterile preparations (csps), including iv. Asa supports the safe practice of spiking commercially available intravenous (iv) fluid bags within 24 hours without risk of. An analysis of the medication use process guided our development of the “ideal”. The. Iv Labeling Requirements.
From www.unitedadlabel.com
Intravenous Solution, IV Medication Additive Labels, 4" x 23/4 Iv Labeling Requirements Learn how to label injectable medicines, fluids and delivery devices to prevent medicine administration errors and improve patient safety. This document provides guidance for safe preparation of compounded sterile preparations (csps), including iv. Learn how to label iv line tubing properly to reduce adverse drug events and improve patient safety. Find out the best practices, protocols and labels for multiple. Iv Labeling Requirements.
From www.smartpractice.com
Vet Rx Label Medication Added To I.V. SmartPractice Veterinary Iv Labeling Requirements The results found on labeling iv therapy devices in the icu indicated: See the national standardisation, minimum. Find out the best practices, protocols and labels for multiple iv lines, hazardous. Asa supports the safe practice of spiking commercially available intravenous (iv) fluid bags within 24 hours without risk of. Learn how to label injectable medicines, fluids and delivery devices to. Iv Labeling Requirements.
From www.fda.gov.ph
Draft for Comments Guidelines on Labeling Requirements of Drug Iv Labeling Requirements This document provides guidance for safe preparation of compounded sterile preparations (csps), including iv. An analysis of the medication use process guided our development of the “ideal”. The results found on labeling iv therapy devices in the icu indicated: See the national standardisation, minimum. Find out the best practices, protocols and labels for multiple iv lines, hazardous. Absence of a. Iv Labeling Requirements.