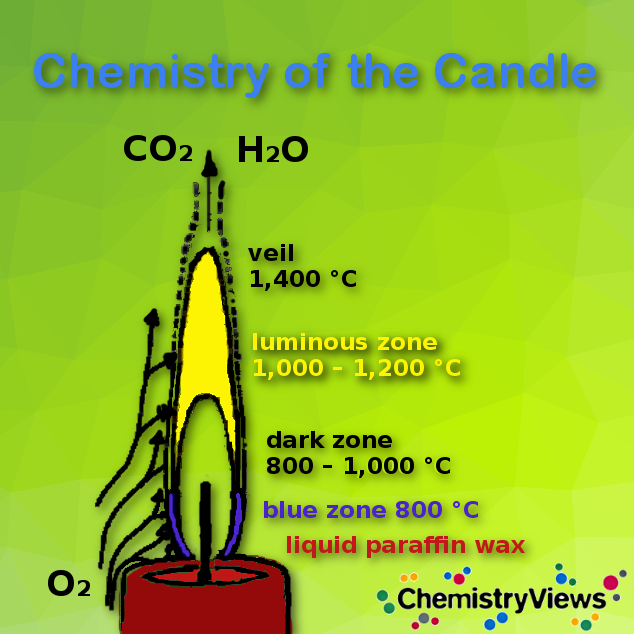
Chemical Formula For Candle Burning . The candle burning process is influenced by a variety of factors, from the type of wax used to the size of the wick, and even the ambient temperature. So, any example of burning you can think of is a combustion reaction, including burning matches, candles, campfires, and gas burners. The equation may look as follows: Chemical reaction of a burning candle: Combustion is also called burning. Rate = k observed (solid wax) x, where k observed = k[o 2] y = constant (equation 3) Also note that water condensation builds. The chemical equation for the combustion of candle wax is as follows: Thus, for a 10 mm candle (90 % paraffin, 10 % stearin), one needs a wick with 24 cotton filaments, but 33 are required for a candle whose diameter is 15 mm. Rate = k(solid wax) x [o 2] y (equation 2) in our lab we will burn the candle in air, in which [o 2] is constant. C 25 h 52 + 38o 2 → 25co 2 + 26h 2 o + energy. The wax needs oxygen from the air to burn. The rate law equation for this reaction is:
from www.chemistryviews.org
C 25 h 52 + 38o 2 → 25co 2 + 26h 2 o + energy. So, any example of burning you can think of is a combustion reaction, including burning matches, candles, campfires, and gas burners. The equation may look as follows: The wax needs oxygen from the air to burn. Thus, for a 10 mm candle (90 % paraffin, 10 % stearin), one needs a wick with 24 cotton filaments, but 33 are required for a candle whose diameter is 15 mm. Also note that water condensation builds. The chemical equation for the combustion of candle wax is as follows: The candle burning process is influenced by a variety of factors, from the type of wax used to the size of the wick, and even the ambient temperature. The rate law equation for this reaction is: Combustion is also called burning.
The Science Behind Candles ChemistryViews
Chemical Formula For Candle Burning Also note that water condensation builds. So, any example of burning you can think of is a combustion reaction, including burning matches, candles, campfires, and gas burners. The candle burning process is influenced by a variety of factors, from the type of wax used to the size of the wick, and even the ambient temperature. C 25 h 52 + 38o 2 → 25co 2 + 26h 2 o + energy. Rate = k observed (solid wax) x, where k observed = k[o 2] y = constant (equation 3) The chemical equation for the combustion of candle wax is as follows: Also note that water condensation builds. Combustion is also called burning. The wax needs oxygen from the air to burn. The equation may look as follows: Thus, for a 10 mm candle (90 % paraffin, 10 % stearin), one needs a wick with 24 cotton filaments, but 33 are required for a candle whose diameter is 15 mm. The rate law equation for this reaction is: Chemical reaction of a burning candle: Rate = k(solid wax) x [o 2] y (equation 2) in our lab we will burn the candle in air, in which [o 2] is constant.
From www.thoughtco.com
An Introduction to Combustion (Burning) Reactions Chemical Formula For Candle Burning Chemical reaction of a burning candle: The equation may look as follows: Rate = k(solid wax) x [o 2] y (equation 2) in our lab we will burn the candle in air, in which [o 2] is constant. Also note that water condensation builds. The candle burning process is influenced by a variety of factors, from the type of wax. Chemical Formula For Candle Burning.
From mekelas.blogspot.com
Combustion Experiment With Candle / function Oxygen In the Combustion simple experiments for Chemical Formula For Candle Burning The candle burning process is influenced by a variety of factors, from the type of wax used to the size of the wick, and even the ambient temperature. Also note that water condensation builds. Rate = k(solid wax) x [o 2] y (equation 2) in our lab we will burn the candle in air, in which [o 2] is constant.. Chemical Formula For Candle Burning.
From www.slideserve.com
PPT UNIT 1 FIRE DYNAMICS PowerPoint Presentation, free download ID3705933 Chemical Formula For Candle Burning The candle burning process is influenced by a variety of factors, from the type of wax used to the size of the wick, and even the ambient temperature. Thus, for a 10 mm candle (90 % paraffin, 10 % stearin), one needs a wick with 24 cotton filaments, but 33 are required for a candle whose diameter is 15 mm.. Chemical Formula For Candle Burning.
From edwinrtwoodard.blogspot.com
Chemical Properties of Candle Wax EdwinrtWoodard Chemical Formula For Candle Burning Rate = k observed (solid wax) x, where k observed = k[o 2] y = constant (equation 3) The chemical equation for the combustion of candle wax is as follows: The equation may look as follows: So, any example of burning you can think of is a combustion reaction, including burning matches, candles, campfires, and gas burners. The wax needs. Chemical Formula For Candle Burning.
From smleo.com
How Do Candles Burn? A Chemistry Annotated Diagram « LEO Chemical Formula For Candle Burning The wax needs oxygen from the air to burn. Combustion is also called burning. Rate = k observed (solid wax) x, where k observed = k[o 2] y = constant (equation 3) So, any example of burning you can think of is a combustion reaction, including burning matches, candles, campfires, and gas burners. Chemical reaction of a burning candle: Also. Chemical Formula For Candle Burning.
From www.slideserve.com
PPT Physical and Chemical Changes PowerPoint Presentation, free download ID9251690 Chemical Formula For Candle Burning The chemical equation for the combustion of candle wax is as follows: Thus, for a 10 mm candle (90 % paraffin, 10 % stearin), one needs a wick with 24 cotton filaments, but 33 are required for a candle whose diameter is 15 mm. Chemical reaction of a burning candle: C 25 h 52 + 38o 2 → 25co 2. Chemical Formula For Candle Burning.
From edwinrtwoodard.blogspot.com
Chemical Properties of Candle Wax EdwinrtWoodard Chemical Formula For Candle Burning The chemical equation for the combustion of candle wax is as follows: Combustion is also called burning. Rate = k(solid wax) x [o 2] y (equation 2) in our lab we will burn the candle in air, in which [o 2] is constant. Also note that water condensation builds. The equation may look as follows: The wax needs oxygen from. Chemical Formula For Candle Burning.
From www.youtube.com
How to Balance C25H52 + O2 = CO2 + H2O (Wax + Oxygen gas ) YouTube Chemical Formula For Candle Burning C 25 h 52 + 38o 2 → 25co 2 + 26h 2 o + energy. Thus, for a 10 mm candle (90 % paraffin, 10 % stearin), one needs a wick with 24 cotton filaments, but 33 are required for a candle whose diameter is 15 mm. Also note that water condensation builds. Combustion is also called burning. The. Chemical Formula For Candle Burning.
From www.slideshare.net
simple chemical reactions chemistry Chemical Formula For Candle Burning Chemical reaction of a burning candle: C 25 h 52 + 38o 2 → 25co 2 + 26h 2 o + energy. So, any example of burning you can think of is a combustion reaction, including burning matches, candles, campfires, and gas burners. Also note that water condensation builds. The candle burning process is influenced by a variety of factors,. Chemical Formula For Candle Burning.
From letstalkscience.ca
Types of Chemical Reactions Let's Talk Science Chemical Formula For Candle Burning The wax needs oxygen from the air to burn. The equation may look as follows: The chemical equation for the combustion of candle wax is as follows: The rate law equation for this reaction is: Thus, for a 10 mm candle (90 % paraffin, 10 % stearin), one needs a wick with 24 cotton filaments, but 33 are required for. Chemical Formula For Candle Burning.
From www.britannica.com
How a Candle Flame Burns Britannica Chemical Formula For Candle Burning Rate = k(solid wax) x [o 2] y (equation 2) in our lab we will burn the candle in air, in which [o 2] is constant. C 25 h 52 + 38o 2 → 25co 2 + 26h 2 o + energy. The candle burning process is influenced by a variety of factors, from the type of wax used to. Chemical Formula For Candle Burning.
From learningarticlez.blogspot.com
The Chemical Change in a Candle Learning Articles Chemical Formula For Candle Burning Combustion is also called burning. Rate = k(solid wax) x [o 2] y (equation 2) in our lab we will burn the candle in air, in which [o 2] is constant. The chemical equation for the combustion of candle wax is as follows: So, any example of burning you can think of is a combustion reaction, including burning matches, candles,. Chemical Formula For Candle Burning.
From www.toppr.com
Metal compound 'A' reacts with dilute sulphuric acid to produce a gas which extinguishes a Chemical Formula For Candle Burning The rate law equation for this reaction is: So, any example of burning you can think of is a combustion reaction, including burning matches, candles, campfires, and gas burners. The chemical equation for the combustion of candle wax is as follows: The candle burning process is influenced by a variety of factors, from the type of wax used to the. Chemical Formula For Candle Burning.
From www.candlescience.com
How To Conduct a Basic Burn Test CandleScience Chemical Formula For Candle Burning C 25 h 52 + 38o 2 → 25co 2 + 26h 2 o + energy. Combustion is also called burning. Chemical reaction of a burning candle: Also note that water condensation builds. Thus, for a 10 mm candle (90 % paraffin, 10 % stearin), one needs a wick with 24 cotton filaments, but 33 are required for a candle. Chemical Formula For Candle Burning.
From byjus.com
Make a labelled diagram of a candle flame. Chemical Formula For Candle Burning Rate = k observed (solid wax) x, where k observed = k[o 2] y = constant (equation 3) The wax needs oxygen from the air to burn. Rate = k(solid wax) x [o 2] y (equation 2) in our lab we will burn the candle in air, in which [o 2] is constant. Also note that water condensation builds. The. Chemical Formula For Candle Burning.
From www.chegg.com
A Burning Candle The FisherKolmogorov equation is a Chemical Formula For Candle Burning Rate = k observed (solid wax) x, where k observed = k[o 2] y = constant (equation 3) The candle burning process is influenced by a variety of factors, from the type of wax used to the size of the wick, and even the ambient temperature. So, any example of burning you can think of is a combustion reaction, including. Chemical Formula For Candle Burning.
From trangtraigarung.com
Do 2 Wick Candles Burn Faster? Unveiling The Truth Behind Twin Flames Chemical Formula For Candle Burning Rate = k observed (solid wax) x, where k observed = k[o 2] y = constant (equation 3) Thus, for a 10 mm candle (90 % paraffin, 10 % stearin), one needs a wick with 24 cotton filaments, but 33 are required for a candle whose diameter is 15 mm. The chemical equation for the combustion of candle wax is. Chemical Formula For Candle Burning.
From slideplayer.com
In a combustion reaction, a candle burns using the oxygen in the air. ppt download Chemical Formula For Candle Burning Chemical reaction of a burning candle: Also note that water condensation builds. The wax needs oxygen from the air to burn. The candle burning process is influenced by a variety of factors, from the type of wax used to the size of the wick, and even the ambient temperature. The equation may look as follows: Combustion is also called burning.. Chemical Formula For Candle Burning.
From www.slideshare.net
light a candle science task Chemical Formula For Candle Burning Also note that water condensation builds. Rate = k observed (solid wax) x, where k observed = k[o 2] y = constant (equation 3) Chemical reaction of a burning candle: C 25 h 52 + 38o 2 → 25co 2 + 26h 2 o + energy. The chemical equation for the combustion of candle wax is as follows: The equation. Chemical Formula For Candle Burning.
From www.ingridscience.ca
Candle chemistry ingridscience.ca Chemical Formula For Candle Burning Chemical reaction of a burning candle: The equation may look as follows: Thus, for a 10 mm candle (90 % paraffin, 10 % stearin), one needs a wick with 24 cotton filaments, but 33 are required for a candle whose diameter is 15 mm. C 25 h 52 + 38o 2 → 25co 2 + 26h 2 o + energy.. Chemical Formula For Candle Burning.
From www.chemistryviews.org
The Science Behind Candles ChemistryViews Chemical Formula For Candle Burning Rate = k observed (solid wax) x, where k observed = k[o 2] y = constant (equation 3) Combustion is also called burning. Thus, for a 10 mm candle (90 % paraffin, 10 % stearin), one needs a wick with 24 cotton filaments, but 33 are required for a candle whose diameter is 15 mm. The rate law equation for. Chemical Formula For Candle Burning.
From www.slideserve.com
PPT Physical and Chemical Changes PowerPoint Presentation, free download ID2756376 Chemical Formula For Candle Burning The candle burning process is influenced by a variety of factors, from the type of wax used to the size of the wick, and even the ambient temperature. Thus, for a 10 mm candle (90 % paraffin, 10 % stearin), one needs a wick with 24 cotton filaments, but 33 are required for a candle whose diameter is 15 mm.. Chemical Formula For Candle Burning.
From www.numerade.com
SOLVED Paraffin, a wax used to make candles, has a formula of C25H52. a. Write the balanced Chemical Formula For Candle Burning The candle burning process is influenced by a variety of factors, from the type of wax used to the size of the wick, and even the ambient temperature. Thus, for a 10 mm candle (90 % paraffin, 10 % stearin), one needs a wick with 24 cotton filaments, but 33 are required for a candle whose diameter is 15 mm.. Chemical Formula For Candle Burning.
From www.youtube.com
Lecture Three The Chemical History of a Candle Products of Combustion (4/6) YouTube Chemical Formula For Candle Burning The equation may look as follows: The chemical equation for the combustion of candle wax is as follows: Combustion is also called burning. Rate = k(solid wax) x [o 2] y (equation 2) in our lab we will burn the candle in air, in which [o 2] is constant. The wax needs oxygen from the air to burn. Rate =. Chemical Formula For Candle Burning.
From www.slideserve.com
PPT Physical and Chemical Changes PowerPoint Presentation, free download ID2756376 Chemical Formula For Candle Burning The chemical equation for the combustion of candle wax is as follows: The rate law equation for this reaction is: The wax needs oxygen from the air to burn. Chemical reaction of a burning candle: Also note that water condensation builds. So, any example of burning you can think of is a combustion reaction, including burning matches, candles, campfires, and. Chemical Formula For Candle Burning.
From www.chemistryviews.org
The Science Behind Candles ChemistryViews Chemical Formula For Candle Burning The chemical equation for the combustion of candle wax is as follows: So, any example of burning you can think of is a combustion reaction, including burning matches, candles, campfires, and gas burners. Chemical reaction of a burning candle: C 25 h 52 + 38o 2 → 25co 2 + 26h 2 o + energy. The candle burning process is. Chemical Formula For Candle Burning.
From slidetodoc.com
Physical and Chemical Changes Ch6 ClassVII Activity 6 Chemical Formula For Candle Burning So, any example of burning you can think of is a combustion reaction, including burning matches, candles, campfires, and gas burners. Rate = k(solid wax) x [o 2] y (equation 2) in our lab we will burn the candle in air, in which [o 2] is constant. The rate law equation for this reaction is: Rate = k observed (solid. Chemical Formula For Candle Burning.
From www.youtube.com
Burning of candle Physical Change or Chemical change class 7 Heat heat basicscience class7 Chemical Formula For Candle Burning The candle burning process is influenced by a variety of factors, from the type of wax used to the size of the wick, and even the ambient temperature. So, any example of burning you can think of is a combustion reaction, including burning matches, candles, campfires, and gas burners. Rate = k(solid wax) x [o 2] y (equation 2) in. Chemical Formula For Candle Burning.
From www.youtube.com
Burning Candle Chemical Reaction YouTube Chemical Formula For Candle Burning The candle burning process is influenced by a variety of factors, from the type of wax used to the size of the wick, and even the ambient temperature. The wax needs oxygen from the air to burn. Chemical reaction of a burning candle: Rate = k observed (solid wax) x, where k observed = k[o 2] y = constant (equation. Chemical Formula For Candle Burning.
From edu.rsc.org
Simple chemical reactions how do candles work? 1114 years Lesson plan RSC Education Chemical Formula For Candle Burning The equation may look as follows: The chemical equation for the combustion of candle wax is as follows: The candle burning process is influenced by a variety of factors, from the type of wax used to the size of the wick, and even the ambient temperature. The wax needs oxygen from the air to burn. C 25 h 52 +. Chemical Formula For Candle Burning.
From www.quora.com
What is the chemical equation reaction) for a candle burning? Quora Chemical Formula For Candle Burning C 25 h 52 + 38o 2 → 25co 2 + 26h 2 o + energy. The chemical equation for the combustion of candle wax is as follows: The wax needs oxygen from the air to burn. Also note that water condensation builds. Rate = k observed (solid wax) x, where k observed = k[o 2] y = constant (equation. Chemical Formula For Candle Burning.
From www.teachoo.com
Structure of a Candle Flame 3 Zones and their hotness Teachoo Chemical Formula For Candle Burning Also note that water condensation builds. Rate = k observed (solid wax) x, where k observed = k[o 2] y = constant (equation 3) The candle burning process is influenced by a variety of factors, from the type of wax used to the size of the wick, and even the ambient temperature. The wax needs oxygen from the air to. Chemical Formula For Candle Burning.
From www.docsity.com
Properties of Matter Burning Candles Introductory Chemistry I CHEM 1405 Docsity Chemical Formula For Candle Burning The wax needs oxygen from the air to burn. The equation may look as follows: The chemical equation for the combustion of candle wax is as follows: The rate law equation for this reaction is: Rate = k(solid wax) x [o 2] y (equation 2) in our lab we will burn the candle in air, in which [o 2] is. Chemical Formula For Candle Burning.
From devan-bloghuang.blogspot.com
Chemical Properties of Candle Wax Chemical Formula For Candle Burning The chemical equation for the combustion of candle wax is as follows: The equation may look as follows: The candle burning process is influenced by a variety of factors, from the type of wax used to the size of the wick, and even the ambient temperature. Chemical reaction of a burning candle: Also note that water condensation builds. C 25. Chemical Formula For Candle Burning.
From www.numerade.com
SOLVED Why are burning candles and rusting nails examples of chemical change? Both form new Chemical Formula For Candle Burning Combustion is also called burning. Thus, for a 10 mm candle (90 % paraffin, 10 % stearin), one needs a wick with 24 cotton filaments, but 33 are required for a candle whose diameter is 15 mm. Rate = k observed (solid wax) x, where k observed = k[o 2] y = constant (equation 3) So, any example of burning. Chemical Formula For Candle Burning.