How To Find Isotopes Of Chlorine . See examples of monatomic and diatomic elements, such as gallium and chlorine. Chlorine (cl) has isotopes with mass numbers ranging from 32 g mol −1 to 40 g mol −1. 53 rows learn about the two stable isotopes of chlorine, 35 cl and 37 cl, and their natural abundances, decay modes, and applications. Learn how to calculate atomic weight from percent abundance of isotopes and how to distinguish between atomic number, mass number and atomic weight. Learn how to find the masses and abundances of isotopes of an element from its mass spectrum. Find out the stable and unstable isotopes of chlorine, its electron configuration and common compounds. Learn about isotopes, the atoms of the same element with different numbers of neutrons. Learn about isotopes, atoms of the same element with different numbers of neutrons, and how they affect relative atomic mass. Find out that chlorine has two stable. There are two principal stable isotopes, 35 cl (75.77%).
from www.alamy.com
Find out the stable and unstable isotopes of chlorine, its electron configuration and common compounds. Find out that chlorine has two stable. Learn how to calculate atomic weight from percent abundance of isotopes and how to distinguish between atomic number, mass number and atomic weight. Learn how to find the masses and abundances of isotopes of an element from its mass spectrum. 53 rows learn about the two stable isotopes of chlorine, 35 cl and 37 cl, and their natural abundances, decay modes, and applications. Chlorine (cl) has isotopes with mass numbers ranging from 32 g mol −1 to 40 g mol −1. Learn about isotopes, the atoms of the same element with different numbers of neutrons. There are two principal stable isotopes, 35 cl (75.77%). Learn about isotopes, atoms of the same element with different numbers of neutrons, and how they affect relative atomic mass. See examples of monatomic and diatomic elements, such as gallium and chlorine.
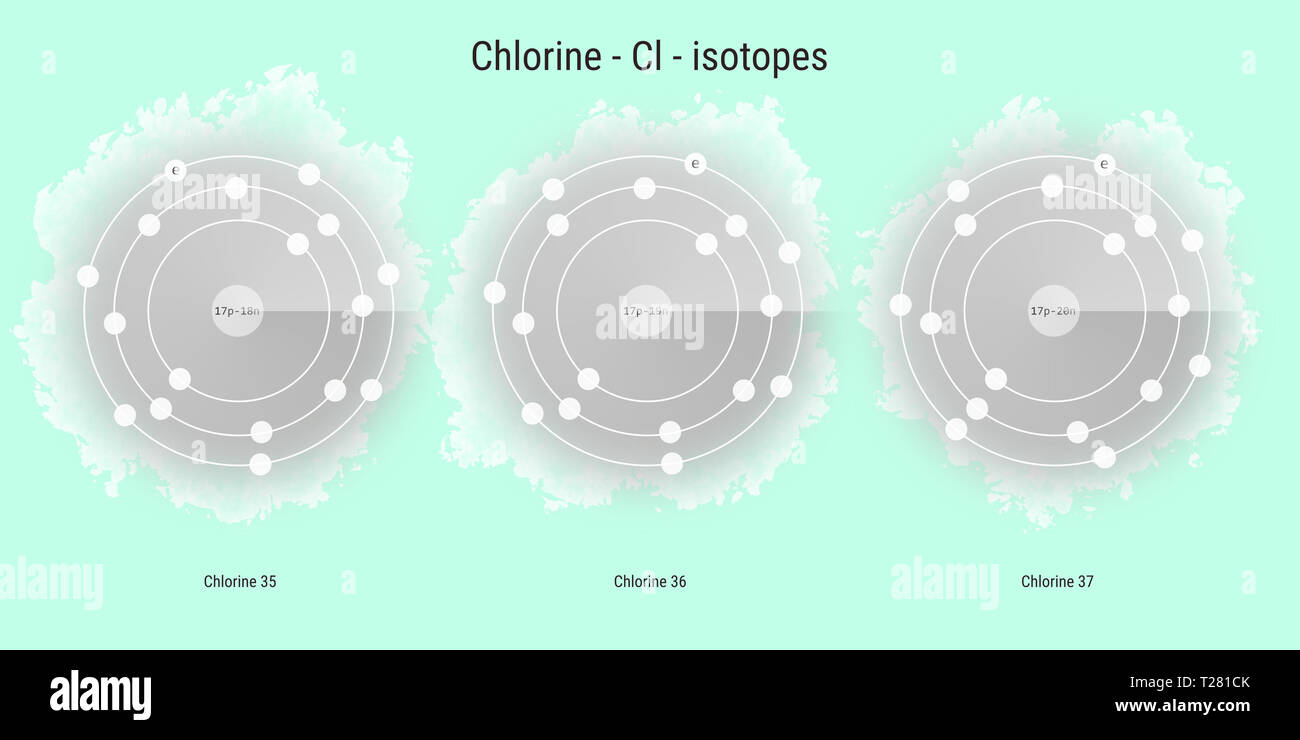
Chlorine chemical element isotopes atomic structure illustration
How To Find Isotopes Of Chlorine There are two principal stable isotopes, 35 cl (75.77%). Chlorine (cl) has isotopes with mass numbers ranging from 32 g mol −1 to 40 g mol −1. Learn how to calculate atomic weight from percent abundance of isotopes and how to distinguish between atomic number, mass number and atomic weight. See examples of monatomic and diatomic elements, such as gallium and chlorine. Learn about isotopes, atoms of the same element with different numbers of neutrons, and how they affect relative atomic mass. 53 rows learn about the two stable isotopes of chlorine, 35 cl and 37 cl, and their natural abundances, decay modes, and applications. There are two principal stable isotopes, 35 cl (75.77%). Find out that chlorine has two stable. Learn about isotopes, the atoms of the same element with different numbers of neutrons. Learn how to find the masses and abundances of isotopes of an element from its mass spectrum. Find out the stable and unstable isotopes of chlorine, its electron configuration and common compounds.
From studiousguy.com
Chlorine (Cl) Properties & Uses StudiousGuy How To Find Isotopes Of Chlorine Learn about isotopes, the atoms of the same element with different numbers of neutrons. Learn how to calculate atomic weight from percent abundance of isotopes and how to distinguish between atomic number, mass number and atomic weight. Learn about isotopes, atoms of the same element with different numbers of neutrons, and how they affect relative atomic mass. 53 rows learn. How To Find Isotopes Of Chlorine.
From www.alamy.com
Isotopes of chlorine. Illustration showing the two principal stable How To Find Isotopes Of Chlorine Learn about isotopes, atoms of the same element with different numbers of neutrons, and how they affect relative atomic mass. See examples of monatomic and diatomic elements, such as gallium and chlorine. Chlorine (cl) has isotopes with mass numbers ranging from 32 g mol −1 to 40 g mol −1. Learn about isotopes, the atoms of the same element with. How To Find Isotopes Of Chlorine.
From www.youtube.com
Isotopes of chlorine (Part 21), General Science Class 8 to 12, MTSE How To Find Isotopes Of Chlorine Learn how to find the masses and abundances of isotopes of an element from its mass spectrum. There are two principal stable isotopes, 35 cl (75.77%). See examples of monatomic and diatomic elements, such as gallium and chlorine. Learn how to calculate atomic weight from percent abundance of isotopes and how to distinguish between atomic number, mass number and atomic. How To Find Isotopes Of Chlorine.
From www.studypool.com
SOLUTION Isotopes isotopes of hydrogen carbon chlorine uranium uses of How To Find Isotopes Of Chlorine Learn about isotopes, the atoms of the same element with different numbers of neutrons. 53 rows learn about the two stable isotopes of chlorine, 35 cl and 37 cl, and their natural abundances, decay modes, and applications. Learn how to find the masses and abundances of isotopes of an element from its mass spectrum. Chlorine (cl) has isotopes with mass. How To Find Isotopes Of Chlorine.
From aqcwfhudny.blogspot.com
How To Find Average Mass Of Isotopes U must byheart the atomic masses How To Find Isotopes Of Chlorine Learn how to find the masses and abundances of isotopes of an element from its mass spectrum. 53 rows learn about the two stable isotopes of chlorine, 35 cl and 37 cl, and their natural abundances, decay modes, and applications. Find out that chlorine has two stable. There are two principal stable isotopes, 35 cl (75.77%). Chlorine (cl) has isotopes. How To Find Isotopes Of Chlorine.
From www.nuclear-power.com
Chlorine Atomic Number Atomic Mass Density of Chlorine nuclear How To Find Isotopes Of Chlorine Find out the stable and unstable isotopes of chlorine, its electron configuration and common compounds. Chlorine (cl) has isotopes with mass numbers ranging from 32 g mol −1 to 40 g mol −1. Learn about isotopes, atoms of the same element with different numbers of neutrons, and how they affect relative atomic mass. Learn how to find the masses and. How To Find Isotopes Of Chlorine.
From www.alamy.com
Chlorine chemical element isotopes atomic structure illustration How To Find Isotopes Of Chlorine Chlorine (cl) has isotopes with mass numbers ranging from 32 g mol −1 to 40 g mol −1. Learn how to find the masses and abundances of isotopes of an element from its mass spectrum. Learn about isotopes, the atoms of the same element with different numbers of neutrons. 53 rows learn about the two stable isotopes of chlorine, 35. How To Find Isotopes Of Chlorine.
From aqcwfhudny.blogspot.com
How To Find Average Mass Of Isotopes U must byheart the atomic masses How To Find Isotopes Of Chlorine Learn about isotopes, atoms of the same element with different numbers of neutrons, and how they affect relative atomic mass. Learn about isotopes, the atoms of the same element with different numbers of neutrons. Chlorine (cl) has isotopes with mass numbers ranging from 32 g mol −1 to 40 g mol −1. Learn how to find the masses and abundances. How To Find Isotopes Of Chlorine.
From askfilo.com
Problem 4.2 The two natural isotopes of chlorine, viz. 1735 Cl and 1737.. How To Find Isotopes Of Chlorine See examples of monatomic and diatomic elements, such as gallium and chlorine. Find out the stable and unstable isotopes of chlorine, its electron configuration and common compounds. Learn about isotopes, the atoms of the same element with different numbers of neutrons. Learn about isotopes, atoms of the same element with different numbers of neutrons, and how they affect relative atomic. How To Find Isotopes Of Chlorine.
From www.numerade.com
tzvb question 3 homework answered cl cl cl while the image above shows How To Find Isotopes Of Chlorine Learn how to calculate atomic weight from percent abundance of isotopes and how to distinguish between atomic number, mass number and atomic weight. Find out that chlorine has two stable. Chlorine (cl) has isotopes with mass numbers ranging from 32 g mol −1 to 40 g mol −1. Learn about isotopes, the atoms of the same element with different numbers. How To Find Isotopes Of Chlorine.
From byjus.com
cl 35 and cl 37 are two isotopes of chlorine . if average atomic mass How To Find Isotopes Of Chlorine Learn about isotopes, atoms of the same element with different numbers of neutrons, and how they affect relative atomic mass. 53 rows learn about the two stable isotopes of chlorine, 35 cl and 37 cl, and their natural abundances, decay modes, and applications. Learn how to calculate atomic weight from percent abundance of isotopes and how to distinguish between atomic. How To Find Isotopes Of Chlorine.
From www.chegg.com
Solved While the image above shows you only 3 isotopes of How To Find Isotopes Of Chlorine 53 rows learn about the two stable isotopes of chlorine, 35 cl and 37 cl, and their natural abundances, decay modes, and applications. Chlorine (cl) has isotopes with mass numbers ranging from 32 g mol −1 to 40 g mol −1. Learn how to find the masses and abundances of isotopes of an element from its mass spectrum. See examples. How To Find Isotopes Of Chlorine.
From www.youtube.com
CHEMISTRY 101 Calculating mass of an isotope in a sample YouTube How To Find Isotopes Of Chlorine Find out the stable and unstable isotopes of chlorine, its electron configuration and common compounds. Learn about isotopes, atoms of the same element with different numbers of neutrons, and how they affect relative atomic mass. Learn how to calculate atomic weight from percent abundance of isotopes and how to distinguish between atomic number, mass number and atomic weight. There are. How To Find Isotopes Of Chlorine.
From scienceprism.co.uk
How archaeologists use chemistry to find out about our past Science Prism How To Find Isotopes Of Chlorine Learn about isotopes, atoms of the same element with different numbers of neutrons, and how they affect relative atomic mass. Learn how to find the masses and abundances of isotopes of an element from its mass spectrum. Learn how to calculate atomic weight from percent abundance of isotopes and how to distinguish between atomic number, mass number and atomic weight.. How To Find Isotopes Of Chlorine.
From www.toppr.com
Chlorine has two isotopes of atomic mass units 34.97 and 36.97. The How To Find Isotopes Of Chlorine Find out the stable and unstable isotopes of chlorine, its electron configuration and common compounds. Learn how to find the masses and abundances of isotopes of an element from its mass spectrum. Learn about isotopes, the atoms of the same element with different numbers of neutrons. Find out that chlorine has two stable. 53 rows learn about the two stable. How To Find Isotopes Of Chlorine.
From www.numerade.com
SOLVED An element m form the compound mh3 when reacts with hydrogen How To Find Isotopes Of Chlorine Learn about isotopes, atoms of the same element with different numbers of neutrons, and how they affect relative atomic mass. There are two principal stable isotopes, 35 cl (75.77%). Learn how to calculate atomic weight from percent abundance of isotopes and how to distinguish between atomic number, mass number and atomic weight. Chlorine (cl) has isotopes with mass numbers ranging. How To Find Isotopes Of Chlorine.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo How To Find Isotopes Of Chlorine Find out the stable and unstable isotopes of chlorine, its electron configuration and common compounds. Find out that chlorine has two stable. Learn about isotopes, the atoms of the same element with different numbers of neutrons. Learn about isotopes, atoms of the same element with different numbers of neutrons, and how they affect relative atomic mass. See examples of monatomic. How To Find Isotopes Of Chlorine.
From valenceelectrons.com
Protons, Neutrons, Electrons for Chlorine (Cl, Cl) How To Find Isotopes Of Chlorine Chlorine (cl) has isotopes with mass numbers ranging from 32 g mol −1 to 40 g mol −1. Learn how to find the masses and abundances of isotopes of an element from its mass spectrum. Find out that chlorine has two stable. Learn about isotopes, atoms of the same element with different numbers of neutrons, and how they affect relative. How To Find Isotopes Of Chlorine.
From periodictable.me
How to Calculate Atomic Mass of Isotopes Archives Dynamic Periodic How To Find Isotopes Of Chlorine Learn how to calculate atomic weight from percent abundance of isotopes and how to distinguish between atomic number, mass number and atomic weight. Find out the stable and unstable isotopes of chlorine, its electron configuration and common compounds. Learn about isotopes, the atoms of the same element with different numbers of neutrons. Learn how to find the masses and abundances. How To Find Isotopes Of Chlorine.
From www.slideserve.com
PPT Relative atomic mass PowerPoint Presentation ID5933036 How To Find Isotopes Of Chlorine See examples of monatomic and diatomic elements, such as gallium and chlorine. Find out the stable and unstable isotopes of chlorine, its electron configuration and common compounds. Learn about isotopes, atoms of the same element with different numbers of neutrons, and how they affect relative atomic mass. Find out that chlorine has two stable. Learn about isotopes, the atoms of. How To Find Isotopes Of Chlorine.
From ameblo.jp
Chlorine Electrons exarepec1973のブログ How To Find Isotopes Of Chlorine Find out the stable and unstable isotopes of chlorine, its electron configuration and common compounds. 53 rows learn about the two stable isotopes of chlorine, 35 cl and 37 cl, and their natural abundances, decay modes, and applications. See examples of monatomic and diatomic elements, such as gallium and chlorine. Learn how to calculate atomic weight from percent abundance of. How To Find Isotopes Of Chlorine.
From byjus.com
what is abundance and how to find abundance of isotopes How To Find Isotopes Of Chlorine Learn about isotopes, the atoms of the same element with different numbers of neutrons. See examples of monatomic and diatomic elements, such as gallium and chlorine. Learn how to find the masses and abundances of isotopes of an element from its mass spectrum. Find out the stable and unstable isotopes of chlorine, its electron configuration and common compounds. Learn how. How To Find Isotopes Of Chlorine.
From www.vrogue.co
Solved All Isotopes Of An Element Form The Same Type vrogue.co How To Find Isotopes Of Chlorine Learn about isotopes, the atoms of the same element with different numbers of neutrons. Learn how to find the masses and abundances of isotopes of an element from its mass spectrum. See examples of monatomic and diatomic elements, such as gallium and chlorine. Chlorine (cl) has isotopes with mass numbers ranging from 32 g mol −1 to 40 g mol. How To Find Isotopes Of Chlorine.
From byjus.com
60. If the average atomic mass of chlorine is 35.5, then find the How To Find Isotopes Of Chlorine Find out the stable and unstable isotopes of chlorine, its electron configuration and common compounds. Learn how to find the masses and abundances of isotopes of an element from its mass spectrum. Learn how to calculate atomic weight from percent abundance of isotopes and how to distinguish between atomic number, mass number and atomic weight. Find out that chlorine has. How To Find Isotopes Of Chlorine.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID5568233 How To Find Isotopes Of Chlorine Learn how to find the masses and abundances of isotopes of an element from its mass spectrum. 53 rows learn about the two stable isotopes of chlorine, 35 cl and 37 cl, and their natural abundances, decay modes, and applications. Learn how to calculate atomic weight from percent abundance of isotopes and how to distinguish between atomic number, mass number. How To Find Isotopes Of Chlorine.
From slideplayer.com
III. Masses of Atoms (p ) Atomic Mass Mass Number Isotopes ppt download How To Find Isotopes Of Chlorine Find out the stable and unstable isotopes of chlorine, its electron configuration and common compounds. Chlorine (cl) has isotopes with mass numbers ranging from 32 g mol −1 to 40 g mol −1. 53 rows learn about the two stable isotopes of chlorine, 35 cl and 37 cl, and their natural abundances, decay modes, and applications. Learn about isotopes, atoms. How To Find Isotopes Of Chlorine.
From www.showme.com
Calculating RAM of Cl Science, Chemistry, Isotopes, AS Level ShowMe How To Find Isotopes Of Chlorine Learn about isotopes, the atoms of the same element with different numbers of neutrons. 53 rows learn about the two stable isotopes of chlorine, 35 cl and 37 cl, and their natural abundances, decay modes, and applications. Find out the stable and unstable isotopes of chlorine, its electron configuration and common compounds. Learn how to find the masses and abundances. How To Find Isotopes Of Chlorine.
From www.youtube.com
Chlorine has two isotopes Cl35 and Cl37. Ratio present in nature is 31 How To Find Isotopes Of Chlorine There are two principal stable isotopes, 35 cl (75.77%). Learn about isotopes, atoms of the same element with different numbers of neutrons, and how they affect relative atomic mass. See examples of monatomic and diatomic elements, such as gallium and chlorine. Learn how to find the masses and abundances of isotopes of an element from its mass spectrum. Learn about. How To Find Isotopes Of Chlorine.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo How To Find Isotopes Of Chlorine 53 rows learn about the two stable isotopes of chlorine, 35 cl and 37 cl, and their natural abundances, decay modes, and applications. Learn about isotopes, the atoms of the same element with different numbers of neutrons. Find out the stable and unstable isotopes of chlorine, its electron configuration and common compounds. Learn about isotopes, atoms of the same element. How To Find Isotopes Of Chlorine.
From www.researchgate.net
Stable isotopes of chlorine Download Scientific Diagram How To Find Isotopes Of Chlorine Learn how to calculate atomic weight from percent abundance of isotopes and how to distinguish between atomic number, mass number and atomic weight. Find out that chlorine has two stable. See examples of monatomic and diatomic elements, such as gallium and chlorine. 53 rows learn about the two stable isotopes of chlorine, 35 cl and 37 cl, and their natural. How To Find Isotopes Of Chlorine.
From brainly.in
define isotope and what are the isotopes of carbon, chlorine, hydrogen How To Find Isotopes Of Chlorine Learn how to calculate atomic weight from percent abundance of isotopes and how to distinguish between atomic number, mass number and atomic weight. Find out the stable and unstable isotopes of chlorine, its electron configuration and common compounds. 53 rows learn about the two stable isotopes of chlorine, 35 cl and 37 cl, and their natural abundances, decay modes, and. How To Find Isotopes Of Chlorine.
From www.expii.com
Isotopes — Definition & Overview Expii How To Find Isotopes Of Chlorine 53 rows learn about the two stable isotopes of chlorine, 35 cl and 37 cl, and their natural abundances, decay modes, and applications. See examples of monatomic and diatomic elements, such as gallium and chlorine. Learn how to calculate atomic weight from percent abundance of isotopes and how to distinguish between atomic number, mass number and atomic weight. Find out. How To Find Isotopes Of Chlorine.
From www.studypool.com
SOLUTION Isotopes isotopes of hydrogen carbon chlorine uranium uses of How To Find Isotopes Of Chlorine Learn about isotopes, the atoms of the same element with different numbers of neutrons. There are two principal stable isotopes, 35 cl (75.77%). Learn about isotopes, atoms of the same element with different numbers of neutrons, and how they affect relative atomic mass. Find out the stable and unstable isotopes of chlorine, its electron configuration and common compounds. See examples. How To Find Isotopes Of Chlorine.
From www.alamy.com
chlorine chemical element isotopes atomic structure illustration How To Find Isotopes Of Chlorine See examples of monatomic and diatomic elements, such as gallium and chlorine. Learn about isotopes, atoms of the same element with different numbers of neutrons, and how they affect relative atomic mass. There are two principal stable isotopes, 35 cl (75.77%). 53 rows learn about the two stable isotopes of chlorine, 35 cl and 37 cl, and their natural abundances,. How To Find Isotopes Of Chlorine.
From www.showme.com
Relative abundancies of Chlorine from a mass spectrometer Science How To Find Isotopes Of Chlorine See examples of monatomic and diatomic elements, such as gallium and chlorine. There are two principal stable isotopes, 35 cl (75.77%). 53 rows learn about the two stable isotopes of chlorine, 35 cl and 37 cl, and their natural abundances, decay modes, and applications. Learn how to calculate atomic weight from percent abundance of isotopes and how to distinguish between. How To Find Isotopes Of Chlorine.