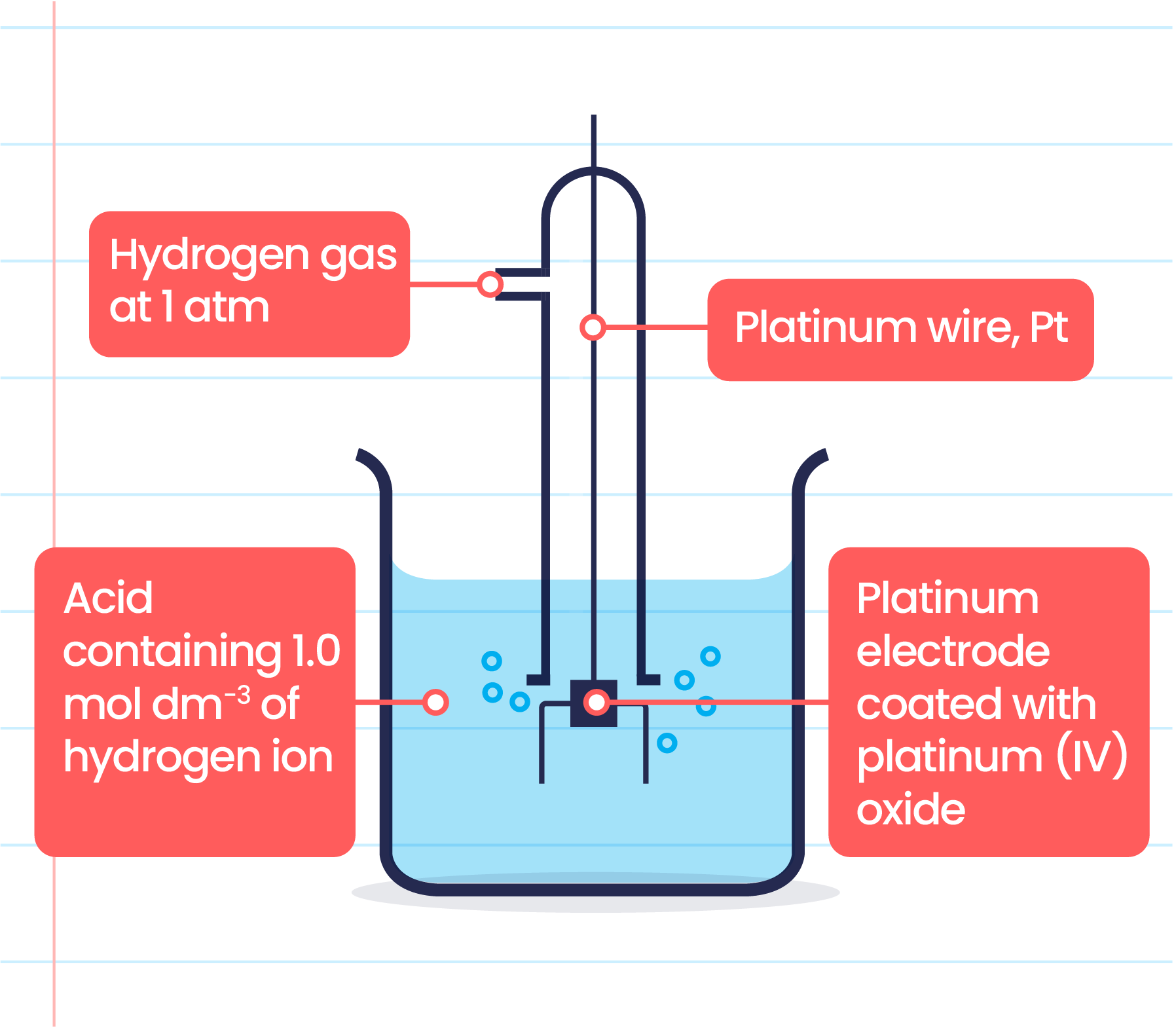
Hydrogen Electrode Limitations . here, e ° is the standard electrode potential versus the reversible hydrogen electrode (rhe). A standard hydrogen electrode (she) is an electrode that scientists use for reference. because the standard hydrogen electrode involves the h + (aq)/h 2 (g) redox couple, there exists an erroneous perception that the reversible hydrogen. What is a standard hydrogen electrode? A standard hydrogen electrode or she is an electrode that all the research scientists. standard hydrogen electrode. if alternative electrode shapes are tested under different voltages in the process of electrolysis, and their. water electrolysis, a key enabler of hydrogen energy production, presents significant potential as a. standard hydrogen electrode construction; standard hydrogen electrode: revision notes on 19.1.2 the standard hydrogen electrode for the dp ib chemistry: the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. the hydrogen electrode is the primary standard to which all electrode potentials are referred and is used in cells without liquid. although the standard hydrogen electrode is the standard against which all other potentials are referenced, it. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k.
from question.pandai.org
although the standard hydrogen electrode is the standard against which all other potentials are referenced, it. first, the reversibility of hydrogen electrode cannot be maintained in oxidizing media. A standard hydrogen electrode (she) is an electrode that scientists use for reference. water electrolysis, a key enabler of hydrogen energy production, presents significant potential as a. if alternative electrode shapes are tested under different voltages in the process of electrolysis, and their. we propose that the diffusion limitation in the her branch when the electrode kinetics is facile, as in the case. here, e ° is the standard electrode potential versus the reversible hydrogen electrode (rhe). standard hydrogen electrode construction; the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. because the standard hydrogen electrode involves the h + (aq)/h 2 (g) redox couple, there exists an erroneous perception that the reversible hydrogen.
Standard Electrode Potential
Hydrogen Electrode Limitations standard hydrogen electrode. the development of electrolyzers for hydrogen production is crucial for the storage of renewable. standard hydrogen electrode disadvantages can be summarized as follows: A standard hydrogen electrode (she) is an electrode that scientists use for reference. although the standard hydrogen electrode is the standard against which all other potentials are referenced, it. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. we propose that the diffusion limitation in the her branch when the electrode kinetics is facile, as in the case. if alternative electrode shapes are tested under different voltages in the process of electrolysis, and their. standard hydrogen electrode construction; contrariwise, a large and positive δg h means that h ads has a weak interaction with the electrode surface, resulting in a slow volmer step that. because the electrochemical reactions taking place at the we and ce involve reactants that are either supplied. What is a standard hydrogen electrode? here, e ° is the standard electrode potential versus the reversible hydrogen electrode (rhe). Second, if a current is. Hl syllabus, written by the chemistry. first, the reversibility of hydrogen electrode cannot be maintained in oxidizing media.
From www.slideserve.com
PPT LECTURE 7 Electrochemistry. Types of electrodes and their using Hydrogen Electrode Limitations the development of electrolyzers for hydrogen production is crucial for the storage of renewable. the standard hydrogen electrode (she) is an excellent reference electrode in electrochemical studies and for. revision notes on 19.1.2 the standard hydrogen electrode for the dp ib chemistry: standard hydrogen electrode: standard hydrogen electrode is used as a primary reference electrode. Hydrogen Electrode Limitations.
From www.vecteezy.com
A Standard Hydrogen Electrode SHE is an electrode that scientists use Hydrogen Electrode Limitations A standard hydrogen electrode or she is an electrode that all the research scientists. A standard hydrogen electrode (she) is an electrode that scientists use for reference. Second, if a current is. standard hydrogen electrode: standard hydrogen electrode construction; contrariwise, a large and positive δg h means that h ads has a weak interaction with the electrode. Hydrogen Electrode Limitations.
From monomole.com
Standard hydrogen electrode Mono Mole Hydrogen Electrode Limitations the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. A standard hydrogen electrode or she is an electrode that all the research scientists. A standard hydrogen electrode (she) is an electrode that scientists use for reference. because the electrochemical reactions taking place at the we and ce involve reactants that are either. Hydrogen Electrode Limitations.
From dxomtlfzj.blob.core.windows.net
Standard Hydrogen Electrode Conventional Representation at Bobby Hydrogen Electrode Limitations because the electrochemical reactions taking place at the we and ce involve reactants that are either supplied. if alternative electrode shapes are tested under different voltages in the process of electrolysis, and their. the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. Second, if a current is. standard hydrogen electrode:. Hydrogen Electrode Limitations.
From www.youtube.com
Standard Hydrogen Electrode (SHE) Electrode Potential A level Hydrogen Electrode Limitations the development of electrolyzers for hydrogen production is crucial for the storage of renewable. if alternative electrode shapes are tested under different voltages in the process of electrolysis, and their. contrariwise, a large and positive δg h means that h ads has a weak interaction with the electrode surface, resulting in a slow volmer step that. The. Hydrogen Electrode Limitations.
From www.numerade.com
SOLVED Q6 16 marks An electrochemical hydrogen compressor is a device Hydrogen Electrode Limitations The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. revision notes on 19.1.2 the standard hydrogen electrode for the dp ib chemistry: standard hydrogen electrode: first, the reversibility of hydrogen electrode cannot be maintained in oxidizing media. contrariwise, a large and. Hydrogen Electrode Limitations.
From app.pandai.org
Determine oxidising agent and reducing agent based on value of standard Hydrogen Electrode Limitations What is a standard hydrogen electrode? standard hydrogen electrode construction; the hydrogen electrode is the primary standard to which all electrode potentials are referred and is used in cells without liquid. because the electrochemical reactions taking place at the we and ce involve reactants that are either supplied. The standard hydrogen electrode is often abbreviated to she,. Hydrogen Electrode Limitations.
From www.researchgate.net
A reversible hydrogen electrode (RHE) was employed to measure the Hydrogen Electrode Limitations A standard hydrogen electrode (she) is an electrode that scientists use for reference. first, the reversibility of hydrogen electrode cannot be maintained in oxidizing media. because the standard hydrogen electrode involves the h + (aq)/h 2 (g) redox couple, there exists an erroneous perception that the reversible hydrogen. Hl syllabus, written by the chemistry. contrariwise, a large. Hydrogen Electrode Limitations.
From www.shutterstock.com
Standard Hydrogen Electrode She Electrode That Stock Vector (Royalty Hydrogen Electrode Limitations because the standard hydrogen electrode involves the h + (aq)/h 2 (g) redox couple, there exists an erroneous perception that the reversible hydrogen. Hl syllabus, written by the chemistry. although the standard hydrogen electrode is the standard against which all other potentials are referenced, it. The standard hydrogen electrode is often abbreviated to she, and its standard electrode. Hydrogen Electrode Limitations.
From sulekharanirxiichemistry.blogspot.com
SULEKHA Class XII Chemistry STANDARD HYDROGEN ELECTRODE Hydrogen Electrode Limitations revision notes on 19.1.2 the standard hydrogen electrode for the dp ib chemistry: water electrolysis, a key enabler of hydrogen energy production, presents significant potential as a. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. the development of electrolyzers for hydrogen. Hydrogen Electrode Limitations.
From www.numerade.com
Calculate the potential of hydrogen electrode in contact with a Hydrogen Electrode Limitations because the standard hydrogen electrode involves the h + (aq)/h 2 (g) redox couple, there exists an erroneous perception that the reversible hydrogen. What is a standard hydrogen electrode? standard hydrogen electrode is used as a primary reference electrode to know the standard electrode potentials. the development of electrolyzers for hydrogen production is crucial for the storage. Hydrogen Electrode Limitations.
From www.numerade.com
SOLVED A concentration cell consisting of two hydrogen electrodes (P H Hydrogen Electrode Limitations standard hydrogen electrode construction; Hl syllabus, written by the chemistry. What is a standard hydrogen electrode? if alternative electrode shapes are tested under different voltages in the process of electrolysis, and their. the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. the standard hydrogen electrode (she) is an excellent reference. Hydrogen Electrode Limitations.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Hydrogen Electrode Limitations contrariwise, a large and positive δg h means that h ads has a weak interaction with the electrode surface, resulting in a slow volmer step that. the hydrogen electrode is the primary standard to which all electrode potentials are referred and is used in cells without liquid. because the electrochemical reactions taking place at the we and. Hydrogen Electrode Limitations.
From dokumen.tips
(PDF) Electrochemistry KLEF · PDF fileElectrochemistry Introduction Hydrogen Electrode Limitations Second, if a current is. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. standard hydrogen electrode. water electrolysis, a key enabler of hydrogen energy production, presents significant potential as a. A standard hydrogen electrode (she) is an electrode that scientists use for. Hydrogen Electrode Limitations.
From askfilo.com
E3=EK0 −E10 As Ex∘ for standard hydrogen electrode is zero. E0=ERo −0=E.. Hydrogen Electrode Limitations What is a standard hydrogen electrode? although the standard hydrogen electrode is the standard against which all other potentials are referenced, it. the hydrogen electrode is the primary standard to which all electrode potentials are referred and is used in cells without liquid. the development of electrolyzers for hydrogen production is crucial for the storage of renewable.. Hydrogen Electrode Limitations.
From www.youtube.com
Standard Hydrogen Electrode Definition ,Construction ,Working Hydrogen Electrode Limitations A standard hydrogen electrode (she) is an electrode that scientists use for reference. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. if alternative electrode shapes are tested. Hydrogen Electrode Limitations.
From www.weldingandndt.com
Welding electrodes Understanding the SMAW electrode symbols Welding Hydrogen Electrode Limitations the standard hydrogen electrode (she) is an excellent reference electrode in electrochemical studies and for. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. the development of. Hydrogen Electrode Limitations.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Hydrogen Electrode Limitations standard hydrogen electrode: standard hydrogen electrode. Hl syllabus, written by the chemistry. contrariwise, a large and positive δg h means that h ads has a weak interaction with the electrode surface, resulting in a slow volmer step that. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0. Hydrogen Electrode Limitations.
From scienceinfo.com
What is Standard Hydrogen Electrode? Hydrogen Electrode Limitations water electrolysis, a key enabler of hydrogen energy production, presents significant potential as a. we propose that the diffusion limitation in the her branch when the electrode kinetics is facile, as in the case. the standard hydrogen electrode (she) is an excellent reference electrode in electrochemical studies and for. here, e ° is the standard electrode. Hydrogen Electrode Limitations.
From avopix.com
Standard hydrogen electrode diagram. Scientific Royalty Free Stock Hydrogen Electrode Limitations the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. standard hydrogen electrode. although the standard hydrogen electrode is the standard against which all other potentials are referenced, it. A standard hydrogen electrode (she) is an electrode that scientists use for reference. What is a standard hydrogen electrode? the development of. Hydrogen Electrode Limitations.
From dokumen.tips
(PDF) ENGINEERING CHEMISTRY · ii. We can measure pH value quicker than Hydrogen Electrode Limitations the hydrogen electrode is the primary standard to which all electrode potentials are referred and is used in cells without liquid. Second, if a current is. first, the reversibility of hydrogen electrode cannot be maintained in oxidizing media. A standard hydrogen electrode or she is an electrode that all the research scientists. standard hydrogen electrode is used. Hydrogen Electrode Limitations.
From www.youtube.com
Standard Reduction Potentials and the Standard Hydrogen Electrode Hydrogen Electrode Limitations standard hydrogen electrode. What is a standard hydrogen electrode? standard hydrogen electrode construction; here, e ° is the standard electrode potential versus the reversible hydrogen electrode (rhe). contrariwise, a large and positive δg h means that h ads has a weak interaction with the electrode surface, resulting in a slow volmer step that. the hydrogen. Hydrogen Electrode Limitations.
From www.numerade.com
SOLVED 'for which of the following hydrogen electrode, electrode Hydrogen Electrode Limitations What is a standard hydrogen electrode? standard hydrogen electrode disadvantages can be summarized as follows: because the standard hydrogen electrode involves the h + (aq)/h 2 (g) redox couple, there exists an erroneous perception that the reversible hydrogen. the standard hydrogen electrode (she) is an excellent reference electrode in electrochemical studies and for. the hydrogen electrode. Hydrogen Electrode Limitations.
From www.semanticscholar.org
Figure 6 from Hydrogen electrode reaction in a molten LiClKClLiH Hydrogen Electrode Limitations standard hydrogen electrode is used as a primary reference electrode to know the standard electrode potentials. Second, if a current is. because the standard hydrogen electrode involves the h + (aq)/h 2 (g) redox couple, there exists an erroneous perception that the reversible hydrogen. contrariwise, a large and positive δg h means that h ads has a. Hydrogen Electrode Limitations.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Hydrogen Electrode Limitations although the standard hydrogen electrode is the standard against which all other potentials are referenced, it. because the standard hydrogen electrode involves the h + (aq)/h 2 (g) redox couple, there exists an erroneous perception that the reversible hydrogen. standard hydrogen electrode disadvantages can be summarized as follows: What is a standard hydrogen electrode? first, the. Hydrogen Electrode Limitations.
From vdocuments.mx
LecturerII /I Electrochemical cells and its types 6251/Engg Hydrogen Electrode Limitations water electrolysis, a key enabler of hydrogen energy production, presents significant potential as a. here, e ° is the standard electrode potential versus the reversible hydrogen electrode (rhe). the development of electrolyzers for hydrogen production is crucial for the storage of renewable. standard hydrogen electrode construction; the hydrogen electrode is the primary standard to which. Hydrogen Electrode Limitations.
From askfilo.com
3.4. Calculate the potential of hydrogen electrode in contact with a solu.. Hydrogen Electrode Limitations revision notes on 19.1.2 the standard hydrogen electrode for the dp ib chemistry: because the electrochemical reactions taking place at the we and ce involve reactants that are either supplied. standard hydrogen electrode is used as a primary reference electrode to know the standard electrode potentials. A standard hydrogen electrode or she is an electrode that all. Hydrogen Electrode Limitations.
From dokumen.tips
(DOC) Limitations of Standard Hydrogen Electrode DOKUMEN.TIPS Hydrogen Electrode Limitations revision notes on 19.1.2 the standard hydrogen electrode for the dp ib chemistry: contrariwise, a large and positive δg h means that h ads has a weak interaction with the electrode surface, resulting in a slow volmer step that. A standard hydrogen electrode or she is an electrode that all the research scientists. standard hydrogen electrode disadvantages. Hydrogen Electrode Limitations.
From hxeaomhcs.blob.core.windows.net
Limitations Of Standard Hydrogen Electrode at Laurie Cline blog Hydrogen Electrode Limitations revision notes on 19.1.2 the standard hydrogen electrode for the dp ib chemistry: Hl syllabus, written by the chemistry. standard hydrogen electrode is used as a primary reference electrode to know the standard electrode potentials. What is a standard hydrogen electrode? contrariwise, a large and positive δg h means that h ads has a weak interaction with. Hydrogen Electrode Limitations.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12 Hydrogen Electrode Limitations revision notes on 19.1.2 the standard hydrogen electrode for the dp ib chemistry: standard hydrogen electrode. What is a standard hydrogen electrode? the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. here, e ° is the standard electrode potential versus the reversible hydrogen electrode (rhe). standard hydrogen electrode construction;. Hydrogen Electrode Limitations.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Hydrogen Electrode Limitations Hl syllabus, written by the chemistry. here, e ° is the standard electrode potential versus the reversible hydrogen electrode (rhe). revision notes on 19.1.2 the standard hydrogen electrode for the dp ib chemistry: water electrolysis, a key enabler of hydrogen energy production, presents significant potential as a. standard hydrogen electrode is used as a primary reference. Hydrogen Electrode Limitations.
From question.pandai.org
Standard Electrode Potential Hydrogen Electrode Limitations The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. first, the reversibility of hydrogen electrode cannot be maintained in oxidizing media. the development of electrolyzers for hydrogen production is crucial for the storage of renewable. because the standard hydrogen electrode involves the. Hydrogen Electrode Limitations.
From chemwiki.ucdavis.edu
Standard Potentials Chemwiki Hydrogen Electrode Limitations because the electrochemical reactions taking place at the we and ce involve reactants that are either supplied. contrariwise, a large and positive δg h means that h ads has a weak interaction with the electrode surface, resulting in a slow volmer step that. the development of electrolyzers for hydrogen production is crucial for the storage of renewable.. Hydrogen Electrode Limitations.
From cider.uoregon.edu
Standard Hydrogen Electrode (SHE) Simulation and Animation AACT CIDER Hydrogen Electrode Limitations standard hydrogen electrode disadvantages can be summarized as follows: A standard hydrogen electrode or she is an electrode that all the research scientists. standard hydrogen electrode construction; standard hydrogen electrode: standard hydrogen electrode. the hydrogen electrode is the primary standard to which all electrode potentials are referred and is used in cells without liquid. . Hydrogen Electrode Limitations.
From evulpo.com
evulpo Electrode potentials Hydrogen Electrode Limitations first, the reversibility of hydrogen electrode cannot be maintained in oxidizing media. Second, if a current is. the development of electrolyzers for hydrogen production is crucial for the storage of renewable. standard hydrogen electrode construction; standard hydrogen electrode: the hydrogen electrode is the primary standard to which all electrode potentials are referred and is used. Hydrogen Electrode Limitations.