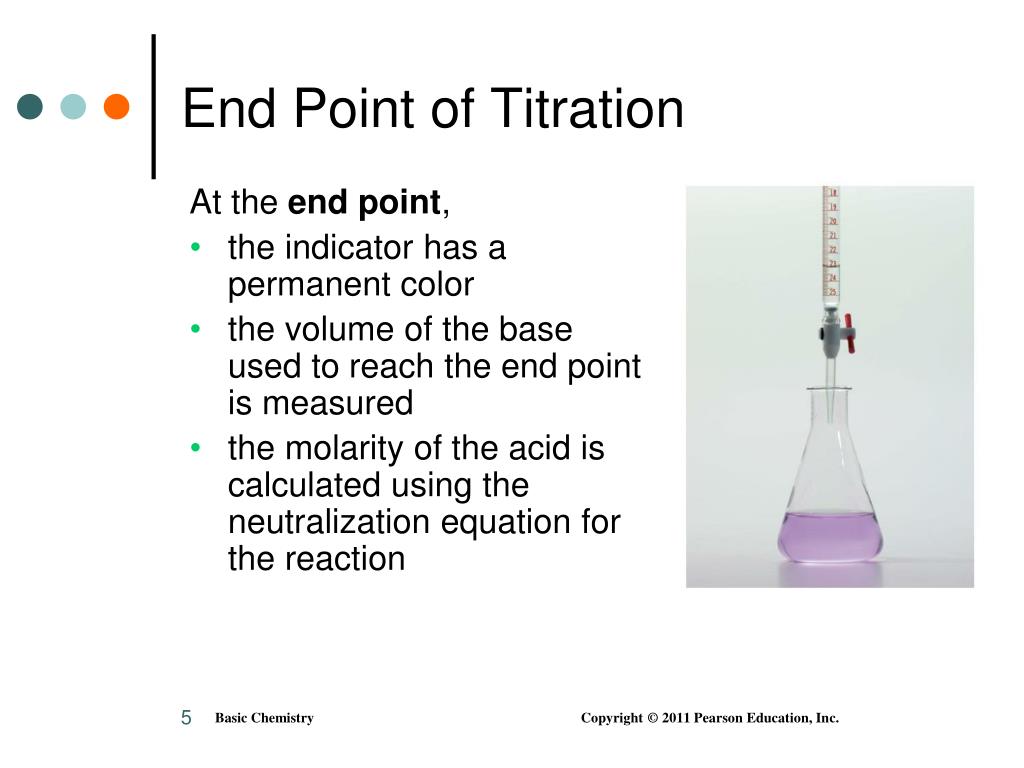
Why Are Different Indicators Used In Titration . indicators are substances whose solutions change color due to changes in ph. in a titration, you determine an unknown concentration of a sample by adding a second reactant of known. choosing the best indicator for different titrations depending on the ph at the equivalence point. These traits are desirable so only a small amount of an indicator is. various indicators have different ionization constants and therefore they show a change in colour at different ph intervals. A useful indicator has a strong color that changes quickly near its pka. methyl orange is one of the indicators commonly used in titrations. how titrations work and what you need. the process of carrying out a titration allows us to accurately measure the volumes of an acid and an alkali required for. You can see that the. Compute sample ph at important stages of a titration. in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. In an alkaline solution, methyl orange is yellow. Every titration uses the same players: revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams.
from exovmncod.blob.core.windows.net
methyl orange is one of the indicators commonly used in titrations. A useful indicator has a strong color that changes quickly near its pka. Compute sample ph at important stages of a titration. Every titration uses the same players: the process of carrying out a titration allows us to accurately measure the volumes of an acid and an alkali required for. You can see that the. indicators are weak organic acids or bases that are different colors in their dissociated and undissociated. various indicators have different ionization constants and therefore they show a change in colour at different ph intervals. indicators are substances whose solutions change color due to changes in ph. Which indicator is used depends on the chemistry of the.
Indicator Definition In A Titration at Maria Little blog
Why Are Different Indicators Used In Titration choosing the best indicator for different titrations depending on the ph at the equivalence point. if you look at the titration curve, which plots the volume of base added vs ph (source): in a titration, you determine an unknown concentration of a sample by adding a second reactant of known. how titrations work and what you need. These traits are desirable so only a small amount of an indicator is. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. Which indicator is used depends on the chemistry of the. Compute sample ph at important stages of a titration. In an alkaline solution, methyl orange is yellow. various indicators have different ionization constants and therefore they show a change in colour at different ph intervals. methyl orange is one of the indicators commonly used in titrations. indicators are weak organic acids or bases that are different colors in their dissociated and undissociated. different indicators can change colour at different ph values, this is why the same indicators aren’t always used for different. A useful indicator has a strong color that changes quickly near its pka. in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. the process of carrying out a titration allows us to accurately measure the volumes of an acid and an alkali required for.
From mungfali.com
Acid Base Titration Indicator Why Are Different Indicators Used In Titration in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. There are many different types of indicators used in titration experiments. different indicators can change colour at different ph values, this is why the same indicators aren’t always used for different. indicators. Why Are Different Indicators Used In Titration.
From mmerevise.co.uk
pH Curves Questions and Revision MME Why Are Different Indicators Used In Titration Every titration uses the same players: A useful indicator has a strong color that changes quickly near its pka. You can see that the. if you look at the titration curve, which plots the volume of base added vs ph (source): methyl orange is one of the indicators commonly used in titrations. how titrations work and what. Why Are Different Indicators Used In Titration.
From saylordotorg.github.io
AcidBase Titrations Why Are Different Indicators Used In Titration choosing the best indicator for different titrations depending on the ph at the equivalence point. indicators are substances whose solutions change color due to changes in ph. in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. in a titration, you. Why Are Different Indicators Used In Titration.
From hxeqgqbgy.blob.core.windows.net
Titration Particle Diagram at Maxine Hatch blog Why Are Different Indicators Used In Titration in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. in a titration, you determine an unknown concentration. Why Are Different Indicators Used In Titration.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector Why Are Different Indicators Used In Titration in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. indicators are weak organic acids or bases that are different colors in their dissociated and undissociated. These traits are desirable so only a small amount of an indicator is. You can see that. Why Are Different Indicators Used In Titration.
From www.vrogue.co
Titration Indicator Types Procedure Indicators vrogue.co Why Are Different Indicators Used In Titration A useful indicator has a strong color that changes quickly near its pka. indicators are weak organic acids or bases that are different colors in their dissociated and undissociated. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. Compute sample ph at important. Why Are Different Indicators Used In Titration.
From monomole.com
Titration overview Mono Mole Why Are Different Indicators Used In Titration indicators are weak organic acids or bases that are different colors in their dissociated and undissociated. if you look at the titration curve, which plots the volume of base added vs ph (source): choosing the best indicator for different titrations depending on the ph at the equivalence point. Compute sample ph at important stages of a titration.. Why Are Different Indicators Used In Titration.
From www.chegg.com
Solved Two students used two different indicators to titrate Why Are Different Indicators Used In Titration different indicators can change colour at different ph values, this is why the same indicators aren’t always used for different. in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. There are many different types of indicators used in titration experiments. Every titration. Why Are Different Indicators Used In Titration.
From dxoiimgcr.blob.core.windows.net
Difference Between Pipet And Volumetric Pipette at Leo Ross blog Why Are Different Indicators Used In Titration if you look at the titration curve, which plots the volume of base added vs ph (source): various indicators have different ionization constants and therefore they show a change in colour at different ph intervals. choosing the best indicator for different titrations depending on the ph at the equivalence point. Which indicator is used depends on the. Why Are Different Indicators Used In Titration.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog Why Are Different Indicators Used In Titration A useful indicator has a strong color that changes quickly near its pka. indicators are weak organic acids or bases that are different colors in their dissociated and undissociated. the process of carrying out a titration allows us to accurately measure the volumes of an acid and an alkali required for. revision notes on 1.7.13 indicators used. Why Are Different Indicators Used In Titration.
From ar.inspiredpencil.com
Titration Phenolphthalein Why Are Different Indicators Used In Titration In an alkaline solution, methyl orange is yellow. Every titration uses the same players: choosing the best indicator for different titrations depending on the ph at the equivalence point. different indicators can change colour at different ph values, this is why the same indicators aren’t always used for different. You can see that the. indicators are substances. Why Are Different Indicators Used In Titration.
From community.asdlib.org
Indicators for Complexation Titrations Image and Video Exchange Why Are Different Indicators Used In Titration Compute sample ph at important stages of a titration. the process of carrying out a titration allows us to accurately measure the volumes of an acid and an alkali required for. A useful indicator has a strong color that changes quickly near its pka. in more basic solutions where the hydronium ion concentration is less than 5.0 ×. Why Are Different Indicators Used In Titration.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog Why Are Different Indicators Used In Titration You can see that the. if you look at the titration curve, which plots the volume of base added vs ph (source): the process of carrying out a titration allows us to accurately measure the volumes of an acid and an alkali required for. These traits are desirable so only a small amount of an indicator is. . Why Are Different Indicators Used In Titration.
From www.chegg.com
Solved 1. Figure 1 shows the titration curves of four Why Are Different Indicators Used In Titration indicators are substances whose solutions change color due to changes in ph. if you look at the titration curve, which plots the volume of base added vs ph (source): A useful indicator has a strong color that changes quickly near its pka. in a titration, you determine an unknown concentration of a sample by adding a second. Why Are Different Indicators Used In Titration.
From www.youtube.com
Precipitation Titrations YouTube Why Are Different Indicators Used In Titration In an alkaline solution, methyl orange is yellow. Compute sample ph at important stages of a titration. how titrations work and what you need. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. in a titration, you determine an unknown concentration of. Why Are Different Indicators Used In Titration.
From pediaa.com
Difference Between AcidBase Titration and Redox Titration Why Are Different Indicators Used In Titration revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. indicators are weak organic acids or bases that are different colors in their dissociated and undissociated. methyl orange is one of the indicators commonly used in titrations. how titrations work and what. Why Are Different Indicators Used In Titration.
From exoxuekni.blob.core.windows.net
Acid Base Titration Uses at Jennifer Baird blog Why Are Different Indicators Used In Titration if you look at the titration curve, which plots the volume of base added vs ph (source): There are many different types of indicators used in titration experiments. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. Compute sample ph at important stages. Why Are Different Indicators Used In Titration.
From courses.lumenlearning.com
AcidBase Titrations Boundless Chemistry Why Are Different Indicators Used In Titration Compute sample ph at important stages of a titration. methyl orange is one of the indicators commonly used in titrations. in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. revision notes on 1.7.13 indicators used in titration for the cie a. Why Are Different Indicators Used In Titration.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog Why Are Different Indicators Used In Titration There are many different types of indicators used in titration experiments. A useful indicator has a strong color that changes quickly near its pka. how titrations work and what you need. if you look at the titration curve, which plots the volume of base added vs ph (source): choosing the best indicator for different titrations depending on. Why Are Different Indicators Used In Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Why Are Different Indicators Used In Titration Which indicator is used depends on the chemistry of the. in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. indicators are substances whose solutions change color due to changes in ph. in a titration, you determine an unknown concentration of a. Why Are Different Indicators Used In Titration.
From chrominfo.blogspot.com
Chrominfo Indicators of complexometric titration Why Are Different Indicators Used In Titration A useful indicator has a strong color that changes quickly near its pka. different indicators can change colour at different ph values, this is why the same indicators aren’t always used for different. various indicators have different ionization constants and therefore they show a change in colour at different ph intervals. the process of carrying out a. Why Are Different Indicators Used In Titration.
From dxofrcamx.blob.core.windows.net
What Is The Meaning Of Indicator In Chemistry at Laura Nelson blog Why Are Different Indicators Used In Titration various indicators have different ionization constants and therefore they show a change in colour at different ph intervals. Which indicator is used depends on the chemistry of the. in a titration, you determine an unknown concentration of a sample by adding a second reactant of known. Every titration uses the same players: A useful indicator has a strong. Why Are Different Indicators Used In Titration.
From dxolxlskr.blob.core.windows.net
Why Use Titration To Find Concentration at Rodney Ruggerio blog Why Are Different Indicators Used In Titration different indicators can change colour at different ph values, this is why the same indicators aren’t always used for different. These traits are desirable so only a small amount of an indicator is. the process of carrying out a titration allows us to accurately measure the volumes of an acid and an alkali required for. In an alkaline. Why Are Different Indicators Used In Titration.
From pressbooks.bccampus.ca
8.7 AcidBase Titrations Chemistry for Chemical Engineers Why Are Different Indicators Used In Titration There are many different types of indicators used in titration experiments. choosing the best indicator for different titrations depending on the ph at the equivalence point. Compute sample ph at important stages of a titration. A useful indicator has a strong color that changes quickly near its pka. various indicators have different ionization constants and therefore they show. Why Are Different Indicators Used In Titration.
From www.microlit.com
An Advanced Guide to Titration Microlit Why Are Different Indicators Used In Titration the process of carrying out a titration allows us to accurately measure the volumes of an acid and an alkali required for. Compute sample ph at important stages of a titration. choosing the best indicator for different titrations depending on the ph at the equivalence point. indicators are weak organic acids or bases that are different colors. Why Are Different Indicators Used In Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Why Are Different Indicators Used In Titration different indicators can change colour at different ph values, this is why the same indicators aren’t always used for different. how titrations work and what you need. Which indicator is used depends on the chemistry of the. indicators are substances whose solutions change color due to changes in ph. In an alkaline solution, methyl orange is yellow.. Why Are Different Indicators Used In Titration.
From hxeqgqbgy.blob.core.windows.net
Titration Particle Diagram at Maxine Hatch blog Why Are Different Indicators Used In Titration Every titration uses the same players: A useful indicator has a strong color that changes quickly near its pka. There are many different types of indicators used in titration experiments. You can see that the. in a titration, you determine an unknown concentration of a sample by adding a second reactant of known. revision notes on 1.7.13 indicators. Why Are Different Indicators Used In Titration.
From dxohctahh.blob.core.windows.net
Examples Of Indicators at Steve Allen blog Why Are Different Indicators Used In Titration Which indicator is used depends on the chemistry of the. In an alkaline solution, methyl orange is yellow. You can see that the. in a titration, you determine an unknown concentration of a sample by adding a second reactant of known. A useful indicator has a strong color that changes quickly near its pka. in more basic solutions. Why Are Different Indicators Used In Titration.
From www.chemistryscl.com
Titrimetry, Titration Classifications, Standard solutions, Equivalence Why Are Different Indicators Used In Titration if you look at the titration curve, which plots the volume of base added vs ph (source): Every titration uses the same players: A useful indicator has a strong color that changes quickly near its pka. how titrations work and what you need. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry. Why Are Different Indicators Used In Titration.
From dxodkszcm.blob.core.windows.net
Titration In Chemistry Is at Laura Fraser blog Why Are Different Indicators Used In Titration These traits are desirable so only a small amount of an indicator is. if you look at the titration curve, which plots the volume of base added vs ph (source): in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. indicators are. Why Are Different Indicators Used In Titration.
From exoluaxpt.blob.core.windows.net
Titration Kid Definition Chemistry at Linda Arguello blog Why Are Different Indicators Used In Titration in a titration, you determine an unknown concentration of a sample by adding a second reactant of known. in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Which indicator is used depends on the chemistry of the. different indicators can change. Why Are Different Indicators Used In Titration.
From wisc.pb.unizin.org
M16Q4 Titration of a Strong Acid with a Strong Base Chem 103/104 Why Are Different Indicators Used In Titration Compute sample ph at important stages of a titration. Which indicator is used depends on the chemistry of the. the process of carrying out a titration allows us to accurately measure the volumes of an acid and an alkali required for. In an alkaline solution, methyl orange is yellow. indicators are substances whose solutions change color due to. Why Are Different Indicators Used In Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Why Are Different Indicators Used In Titration You can see that the. Compute sample ph at important stages of a titration. various indicators have different ionization constants and therefore they show a change in colour at different ph intervals. in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Every. Why Are Different Indicators Used In Titration.
From exovmncod.blob.core.windows.net
Indicator Definition In A Titration at Maria Little blog Why Are Different Indicators Used In Titration different indicators can change colour at different ph values, this is why the same indicators aren’t always used for different. Compute sample ph at important stages of a titration. indicators are substances whose solutions change color due to changes in ph. indicators are weak organic acids or bases that are different colors in their dissociated and undissociated.. Why Are Different Indicators Used In Titration.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Why Are Different Indicators Used In Titration indicators are weak organic acids or bases that are different colors in their dissociated and undissociated. in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. different indicators can change colour at different ph values, this is why the same indicators aren’t. Why Are Different Indicators Used In Titration.