Magnesium Oxide Enthalpy Of Formation . however, we can apply hess’s law to find the heat of formation for mgo by combining a series of reactions that are much safer. Enthalpy of formation of magnesium oxide objective: in this experiment we will use the experimentally measured enthalpy of reaction for a series of exothermic reactions and hess' law to determine. from there you should be able to use hess' law to calculate the enthalpy change of the formation of magnesium. In this experiment, a simple calorimeter will be constructed and. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of.
from www.progressive-scientific.com
136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. in this experiment we will use the experimentally measured enthalpy of reaction for a series of exothermic reactions and hess' law to determine. In this experiment, a simple calorimeter will be constructed and. from there you should be able to use hess' law to calculate the enthalpy change of the formation of magnesium. however, we can apply hess’s law to find the heat of formation for mgo by combining a series of reactions that are much safer. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Enthalpy of formation of magnesium oxide objective:
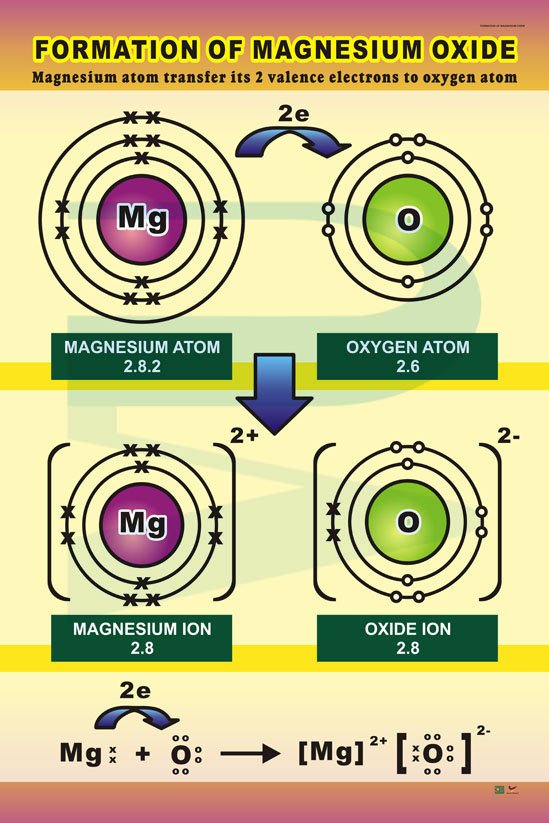
Formation Of Magnesium Oxide Progressive Scientific Sdn. Bhd.
Magnesium Oxide Enthalpy Of Formation however, we can apply hess’s law to find the heat of formation for mgo by combining a series of reactions that are much safer. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. in this experiment we will use the experimentally measured enthalpy of reaction for a series of exothermic reactions and hess' law to determine. however, we can apply hess’s law to find the heat of formation for mgo by combining a series of reactions that are much safer. Enthalpy of formation of magnesium oxide objective: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. In this experiment, a simple calorimeter will be constructed and. from there you should be able to use hess' law to calculate the enthalpy change of the formation of magnesium.
From www.scribd.com
Determining the Enthalpy of Formation of Magnesium Oxide through a Magnesium Oxide Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. In this experiment, a simple calorimeter will be constructed and. in this experiment we will use the experimentally measured enthalpy of reaction for a series of exothermic reactions and hess' law to determine. from there you should be able to. Magnesium Oxide Enthalpy Of Formation.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Magnesium Oxide Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. however, we can apply hess’s law to find the heat of formation for mgo by combining a series of reactions that are much safer. Enthalpy of formation of magnesium oxide objective: in this experiment we will use the experimentally measured. Magnesium Oxide Enthalpy Of Formation.
From thesisfinance.web.fc2.com
A lab experiment heat of reaction for the combustion of magnesium Magnesium Oxide Enthalpy Of Formation however, we can apply hess’s law to find the heat of formation for mgo by combining a series of reactions that are much safer. Enthalpy of formation of magnesium oxide objective: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. In this experiment, a simple calorimeter will be constructed and.. Magnesium Oxide Enthalpy Of Formation.
From dokumen.tips
(PDF) Enthalpy of Formation of Magnesium Oxide 111 Lab/labs/Enthalpy Magnesium Oxide Enthalpy Of Formation in this experiment we will use the experimentally measured enthalpy of reaction for a series of exothermic reactions and hess' law to determine. from there you should be able to use hess' law to calculate the enthalpy change of the formation of magnesium. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Magnesium Oxide Enthalpy Of Formation.
From dokumen.tips
(PDF) Enthalpy of Formation of Magnesium Oxide Colby · PDF Magnesium Oxide Enthalpy Of Formation In this experiment, a simple calorimeter will be constructed and. in this experiment we will use the experimentally measured enthalpy of reaction for a series of exothermic reactions and hess' law to determine. however, we can apply hess’s law to find the heat of formation for mgo by combining a series of reactions that are much safer. . Magnesium Oxide Enthalpy Of Formation.
From www.youtube.com
The Formation of Magnesium Oxide YouTube Magnesium Oxide Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. however, we can apply hess’s law to find the heat of formation for mgo by combining a series of reactions that are much safer. Enthalpy of formation of magnesium oxide objective: In this experiment, a simple calorimeter will be constructed and.. Magnesium Oxide Enthalpy Of Formation.
From oneclass.com
OneClass 20. Using the data below, calculate the lattice energy of Magnesium Oxide Enthalpy Of Formation 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. In this experiment, a simple calorimeter will be constructed and. Enthalpy of formation of magnesium oxide objective: from there you should be able to use hess' law to calculate the enthalpy change of the formation of magnesium. 193. Magnesium Oxide Enthalpy Of Formation.
From www.youtube.com
Enthalpy of Formation of Magnesium Oxide YouTube Magnesium Oxide Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. in this experiment we will use the experimentally measured enthalpy of reaction for a series of exothermic reactions and hess' law. Magnesium Oxide Enthalpy Of Formation.
From www.numerade.com
SOLVED The known standard enthalpy of formation for magnesium oxide is Magnesium Oxide Enthalpy Of Formation In this experiment, a simple calorimeter will be constructed and. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. in this experiment we will use the experimentally measured enthalpy of reaction for a series of exothermic reactions and hess' law to determine. Enthalpy of formation of magnesium oxide. Magnesium Oxide Enthalpy Of Formation.
From docslib.org
Experiment 6 Using Calorimetry to Determine the Enthalpy of Formation Magnesium Oxide Enthalpy Of Formation however, we can apply hess’s law to find the heat of formation for mgo by combining a series of reactions that are much safer. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. Enthalpy of formation of magnesium oxide objective: from there you should be able to. Magnesium Oxide Enthalpy Of Formation.
From www.chegg.com
Delta H(formation) of MgO Note My lab mannual Magnesium Oxide Enthalpy Of Formation 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. from there you should be able to use hess' law to calculate the enthalpy change of the formation of magnesium. In this experiment, a simple calorimeter will be constructed and. in this experiment we will use the experimentally. Magnesium Oxide Enthalpy Of Formation.
From cupsoguepictures.com
😍 Enthalpy of formation of mgo. magnesium oxide. 20190218 Magnesium Oxide Enthalpy Of Formation in this experiment we will use the experimentally measured enthalpy of reaction for a series of exothermic reactions and hess' law to determine. from there you should be able to use hess' law to calculate the enthalpy change of the formation of magnesium. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Magnesium Oxide Enthalpy Of Formation.
From lectio.info
Heat Of Formation Of Mgo Magnesium Oxide Enthalpy Of Formation Enthalpy of formation of magnesium oxide objective: In this experiment, a simple calorimeter will be constructed and. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. however, we can apply hess’s law to find the heat of formation for mgo by combining a series of reactions that are. Magnesium Oxide Enthalpy Of Formation.
From mavink.com
Standard Enthalpy Chart Magnesium Oxide Enthalpy Of Formation Enthalpy of formation of magnesium oxide objective: however, we can apply hess’s law to find the heat of formation for mgo by combining a series of reactions that are much safer. from there you should be able to use hess' law to calculate the enthalpy change of the formation of magnesium. 136 rows standard enthalpy change of. Magnesium Oxide Enthalpy Of Formation.
From cupsoguepictures.com
😍 Enthalpy of formation of mgo. magnesium oxide. 20190218 Magnesium Oxide Enthalpy Of Formation 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. from there you should be able to use hess' law to calculate the enthalpy change of the formation of magnesium. Enthalpy of formation of magnesium oxide objective: In this experiment, a simple calorimeter will be constructed and. 193. Magnesium Oxide Enthalpy Of Formation.
From talisman-intl.com
🎉 Enthalpy of formation of mgo. Enthalpy of formation of magnesium Magnesium Oxide Enthalpy Of Formation in this experiment we will use the experimentally measured enthalpy of reaction for a series of exothermic reactions and hess' law to determine. however, we can apply hess’s law to find the heat of formation for mgo by combining a series of reactions that are much safer. from there you should be able to use hess' law. Magnesium Oxide Enthalpy Of Formation.
From www.youtube.com
FORMATION OF MAGNESIUM OXIDE YouTube Magnesium Oxide Enthalpy Of Formation in this experiment we will use the experimentally measured enthalpy of reaction for a series of exothermic reactions and hess' law to determine. Enthalpy of formation of magnesium oxide objective: from there you should be able to use hess' law to calculate the enthalpy change of the formation of magnesium. however, we can apply hess’s law to. Magnesium Oxide Enthalpy Of Formation.
From www.youtube.com
Lattice Enthalpy and Born Haber Cycle of Magnesium Oxide YouTube Magnesium Oxide Enthalpy Of Formation in this experiment we will use the experimentally measured enthalpy of reaction for a series of exothermic reactions and hess' law to determine. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Enthalpy of formation of magnesium oxide objective: In this experiment, a simple calorimeter will be constructed and. . Magnesium Oxide Enthalpy Of Formation.
From studylib.net
Exp. 7 Enthalpy of Form ation of Magnesium Oxide Background Magnesium Oxide Enthalpy Of Formation 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. however, we can apply hess’s law to find the heat of formation for mgo by combining a series of reactions that are much safer. in this experiment we will use the experimentally measured enthalpy of reaction for a. Magnesium Oxide Enthalpy Of Formation.
From www.transtutors.com
(Solved) EXPERIMENT 13 THERMODYNAMICS ENTHALPY OF FORMATION Magnesium Oxide Enthalpy Of Formation however, we can apply hess’s law to find the heat of formation for mgo by combining a series of reactions that are much safer. from there you should be able to use hess' law to calculate the enthalpy change of the formation of magnesium. 136 rows standard enthalpy change of formation (data table) these tables include heat. Magnesium Oxide Enthalpy Of Formation.
From www.slideshare.net
Enthalpy of formation of magnesium oxide Magnesium Oxide Enthalpy Of Formation Enthalpy of formation of magnesium oxide objective: in this experiment we will use the experimentally measured enthalpy of reaction for a series of exothermic reactions and hess' law to determine. In this experiment, a simple calorimeter will be constructed and. however, we can apply hess’s law to find the heat of formation for mgo by combining a series. Magnesium Oxide Enthalpy Of Formation.
From www.youtube.com
Lattice Enthalpy and Born Haber Cycle for Magnesium Chloride YouTube Magnesium Oxide Enthalpy Of Formation from there you should be able to use hess' law to calculate the enthalpy change of the formation of magnesium. in this experiment we will use the experimentally measured enthalpy of reaction for a series of exothermic reactions and hess' law to determine. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Magnesium Oxide Enthalpy Of Formation.
From www.transtutors.com
(Solved) Question LAB 9 ENTHALPY OF FORMATION OF MAGNESIUM OXIDE Magnesium Oxide Enthalpy Of Formation from there you should be able to use hess' law to calculate the enthalpy change of the formation of magnesium. In this experiment, a simple calorimeter will be constructed and. in this experiment we will use the experimentally measured enthalpy of reaction for a series of exothermic reactions and hess' law to determine. Enthalpy of formation of magnesium. Magnesium Oxide Enthalpy Of Formation.
From www.slideshare.net
Enthalpy of formation of magnesium oxide Magnesium Oxide Enthalpy Of Formation from there you should be able to use hess' law to calculate the enthalpy change of the formation of magnesium. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. however, we can apply hess’s law to find the heat of formation for mgo by combining a series. Magnesium Oxide Enthalpy Of Formation.
From hollyburton.z19.web.core.windows.net
Enthalpy Of Formation Chart Magnesium Oxide Enthalpy Of Formation Enthalpy of formation of magnesium oxide objective: in this experiment we will use the experimentally measured enthalpy of reaction for a series of exothermic reactions and hess' law to determine. from there you should be able to use hess' law to calculate the enthalpy change of the formation of magnesium. 136 rows standard enthalpy change of formation. Magnesium Oxide Enthalpy Of Formation.
From www.chegg.com
Solved 4. The standard enthalpy of formation of magnesium Magnesium Oxide Enthalpy Of Formation Enthalpy of formation of magnesium oxide objective: In this experiment, a simple calorimeter will be constructed and. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. however, we can apply hess’s law to find the heat of formation for mgo by combining a series of reactions that are. Magnesium Oxide Enthalpy Of Formation.
From studymoose.com
Determine Magnesium Oxide's Standard Enthalpy of Formation with Hess Magnesium Oxide Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. however, we can apply hess’s law to find the heat of formation for mgo by combining a series of reactions that are much safer. In this experiment, a simple calorimeter will be constructed and. Enthalpy of formation of magnesium oxide objective:. Magnesium Oxide Enthalpy Of Formation.
From studylib.net
Experiment 9 Enthalpy of Formation of Magnesium Oxide Magnesium Oxide Enthalpy Of Formation In this experiment, a simple calorimeter will be constructed and. however, we can apply hess’s law to find the heat of formation for mgo by combining a series of reactions that are much safer. from there you should be able to use hess' law to calculate the enthalpy change of the formation of magnesium. 136 rows standard. Magnesium Oxide Enthalpy Of Formation.
From studylib.net
MgO Heat of Reaction Lab Magnesium Oxide Enthalpy Of Formation however, we can apply hess’s law to find the heat of formation for mgo by combining a series of reactions that are much safer. Enthalpy of formation of magnesium oxide objective: in this experiment we will use the experimentally measured enthalpy of reaction for a series of exothermic reactions and hess' law to determine. from there you. Magnesium Oxide Enthalpy Of Formation.
From momentumclubs.org
🎉 Heat of formation of magnesium oxide. Essay about Heat of formation Magnesium Oxide Enthalpy Of Formation however, we can apply hess’s law to find the heat of formation for mgo by combining a series of reactions that are much safer. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. In this experiment, a simple calorimeter will be constructed and. Enthalpy of formation of magnesium. Magnesium Oxide Enthalpy Of Formation.
From www.youtube.com
Formation of Magnesium Oxide (MgO) Chemical Bonding Atomic Magnesium Oxide Enthalpy Of Formation 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. In this experiment, a simple calorimeter will be constructed and. from there you should be able to use hess' law to calculate the enthalpy change of the formation of magnesium. 193 rows in chemistry and thermodynamics, the standard. Magnesium Oxide Enthalpy Of Formation.
From www.numerade.com
SOLVED4 The standard enthalpy of formation of magnesium oxide_ Use Magnesium Oxide Enthalpy Of Formation from there you should be able to use hess' law to calculate the enthalpy change of the formation of magnesium. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. In this experiment, a simple calorimeter will be constructed and. 136 rows standard enthalpy change of formation (data table) these. Magnesium Oxide Enthalpy Of Formation.
From www.progressive-scientific.com
Formation Of Magnesium Oxide Progressive Scientific Sdn. Bhd. Magnesium Oxide Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. however, we can apply hess’s law to find the heat of formation for mgo by combining a series of reactions that are much safer. in this experiment we will use the experimentally measured enthalpy of reaction for a series of. Magnesium Oxide Enthalpy Of Formation.
From www.youtube.com
TRU Chemistry Labs First Year Experiment 11Heat of Formation of Magnesium Oxide Enthalpy Of Formation however, we can apply hess’s law to find the heat of formation for mgo by combining a series of reactions that are much safer. in this experiment we will use the experimentally measured enthalpy of reaction for a series of exothermic reactions and hess' law to determine. In this experiment, a simple calorimeter will be constructed and. Enthalpy. Magnesium Oxide Enthalpy Of Formation.
From www.studocu.com
Enthalpy of Formation of Magnesium Oxide 2 copy Enthalpy of Formation Magnesium Oxide Enthalpy Of Formation however, we can apply hess’s law to find the heat of formation for mgo by combining a series of reactions that are much safer. Enthalpy of formation of magnesium oxide objective: In this experiment, a simple calorimeter will be constructed and. from there you should be able to use hess' law to calculate the enthalpy change of the. Magnesium Oxide Enthalpy Of Formation.