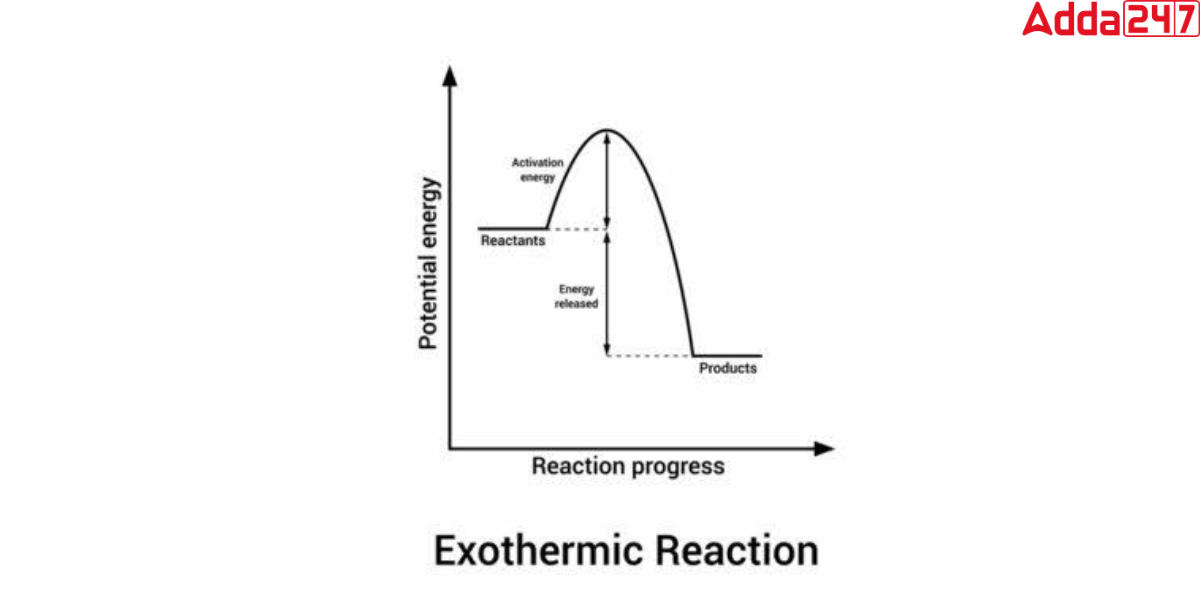
Condensing Steam An Exothermic Reaction . This typically occurs when water vapor molecules. C 12 h 22 o 11 (s) + h 2 so 4 (aq.) + ½ o 2 (g) → 11 c (s) + 12 h 2 o (l) + co 2 (g) + so 2 (g) + heat Condensation is the process by which water vapor turns into liquid water. In the course of an endothermic process, the system gains heat from the. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Sulfuric acid reacts with ordinary table sugar (sucrose) and results in an energetic exothermic reaction. This plot of temperature shows what happens to a 75 g sample of steam initially at 1 atm and 200°c as heat is removed at a constant rate: An exothermic reaction gives off heat energy. Exothermic reactions are reactions that release energy into the environment in the form of heat. Burning wood provides heat through the exothermic chemical reaction of oxygen (o) with cellulose (c 6 h 10 o 5), the major. The reaction produces heat, steam, carbon dioxide (co 2 ), and sulfur dioxide (so 2 ). Exothermic reactions feel warm or hot or may even be.
from www.adda247.com
Sulfuric acid reacts with ordinary table sugar (sucrose) and results in an energetic exothermic reaction. Burning wood provides heat through the exothermic chemical reaction of oxygen (o) with cellulose (c 6 h 10 o 5), the major. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. An exothermic reaction gives off heat energy. C 12 h 22 o 11 (s) + h 2 so 4 (aq.) + ½ o 2 (g) → 11 c (s) + 12 h 2 o (l) + co 2 (g) + so 2 (g) + heat This typically occurs when water vapor molecules. Exothermic reactions feel warm or hot or may even be. Condensation is the process by which water vapor turns into liquid water. This plot of temperature shows what happens to a 75 g sample of steam initially at 1 atm and 200°c as heat is removed at a constant rate:
Exothermic Reaction Examples, Equation, Definition, Meaning
Condensing Steam An Exothermic Reaction C 12 h 22 o 11 (s) + h 2 so 4 (aq.) + ½ o 2 (g) → 11 c (s) + 12 h 2 o (l) + co 2 (g) + so 2 (g) + heat An exothermic reaction gives off heat energy. Exothermic reactions feel warm or hot or may even be. Burning wood provides heat through the exothermic chemical reaction of oxygen (o) with cellulose (c 6 h 10 o 5), the major. In the course of an endothermic process, the system gains heat from the. The reaction produces heat, steam, carbon dioxide (co 2 ), and sulfur dioxide (so 2 ). Exothermic reactions are reactions that release energy into the environment in the form of heat. C 12 h 22 o 11 (s) + h 2 so 4 (aq.) + ½ o 2 (g) → 11 c (s) + 12 h 2 o (l) + co 2 (g) + so 2 (g) + heat Condensation is the process by which water vapor turns into liquid water. Sulfuric acid reacts with ordinary table sugar (sucrose) and results in an energetic exothermic reaction. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. This plot of temperature shows what happens to a 75 g sample of steam initially at 1 atm and 200°c as heat is removed at a constant rate: This typically occurs when water vapor molecules. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings.
From www.coursehero.com
[Solved] A model of an endothermic reaction and an exothermic reaction Condensing Steam An Exothermic Reaction Condensation is the process by which water vapor turns into liquid water. In the course of an endothermic process, the system gains heat from the. Exothermic reactions feel warm or hot or may even be. This typically occurs when water vapor molecules. An exothermic reaction gives off heat energy. A chemical reaction or physical change is endothermic if heat is. Condensing Steam An Exothermic Reaction.
From www.vrogue.co
What Is Exothermic Reaction Exothermic Reactions An E vrogue.co Condensing Steam An Exothermic Reaction An exothermic reaction gives off heat energy. Exothermic reactions feel warm or hot or may even be. In the course of an endothermic process, the system gains heat from the. This typically occurs when water vapor molecules. Condensation is the process by which water vapor turns into liquid water. C 12 h 22 o 11 (s) + h 2 so. Condensing Steam An Exothermic Reaction.
From mavink.com
Exothermic Reaction Condensing Steam An Exothermic Reaction Burning wood provides heat through the exothermic chemical reaction of oxygen (o) with cellulose (c 6 h 10 o 5), the major. In the course of an endothermic process, the system gains heat from the. Exothermic reactions are reactions that release energy into the environment in the form of heat. Sulfuric acid reacts with ordinary table sugar (sucrose) and results. Condensing Steam An Exothermic Reaction.
From www.adda247.com
Exothermic Reaction Examples, Equation, Definition, Meaning Condensing Steam An Exothermic Reaction C 12 h 22 o 11 (s) + h 2 so 4 (aq.) + ½ o 2 (g) → 11 c (s) + 12 h 2 o (l) + co 2 (g) + so 2 (g) + heat Burning wood provides heat through the exothermic chemical reaction of oxygen (o) with cellulose (c 6 h 10 o 5), the major.. Condensing Steam An Exothermic Reaction.
From whatisdiffer.com
Difference Between Endothermic And Exothermic Reactions? Condensing Steam An Exothermic Reaction Exothermic reactions are reactions that release energy into the environment in the form of heat. Sulfuric acid reacts with ordinary table sugar (sucrose) and results in an energetic exothermic reaction. Burning wood provides heat through the exothermic chemical reaction of oxygen (o) with cellulose (c 6 h 10 o 5), the major. Exothermic reactions feel warm or hot or may. Condensing Steam An Exothermic Reaction.
From www.worksheetsplanet.com
What is an Exothermic Reaction Definition and Example Condensing Steam An Exothermic Reaction Burning wood provides heat through the exothermic chemical reaction of oxygen (o) with cellulose (c 6 h 10 o 5), the major. This plot of temperature shows what happens to a 75 g sample of steam initially at 1 atm and 200°c as heat is removed at a constant rate: C 12 h 22 o 11 (s) + h 2. Condensing Steam An Exothermic Reaction.
From quizlet.com
Draw a reaction coordinate diagram for an exothermic reactio Quizlet Condensing Steam An Exothermic Reaction This typically occurs when water vapor molecules. C 12 h 22 o 11 (s) + h 2 so 4 (aq.) + ½ o 2 (g) → 11 c (s) + 12 h 2 o (l) + co 2 (g) + so 2 (g) + heat Burning wood provides heat through the exothermic chemical reaction of oxygen (o) with cellulose (c. Condensing Steam An Exothermic Reaction.
From www.twinkl.com.au
What is a Reaction Profile? Answered Twinkl Twinkl Condensing Steam An Exothermic Reaction Sulfuric acid reacts with ordinary table sugar (sucrose) and results in an energetic exothermic reaction. Exothermic reactions feel warm or hot or may even be. In the course of an endothermic process, the system gains heat from the. The reaction produces heat, steam, carbon dioxide (co 2 ), and sulfur dioxide (so 2 ). Exothermic reactions are reactions that release. Condensing Steam An Exothermic Reaction.
From www.scribd.com
Endothermic Reaction Exothermic Reaction Delta H Delta H PDF Condensing Steam An Exothermic Reaction This typically occurs when water vapor molecules. This plot of temperature shows what happens to a 75 g sample of steam initially at 1 atm and 200°c as heat is removed at a constant rate: In the course of an endothermic process, the system gains heat from the. The reaction produces heat, steam, carbon dioxide (co 2 ), and sulfur. Condensing Steam An Exothermic Reaction.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 3.1.1 Exothermic & Endothermic翰林国际教育 Condensing Steam An Exothermic Reaction A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. The reaction produces heat, steam, carbon dioxide (co 2 ), and sulfur dioxide (so 2 ). This typically occurs when water vapor molecules. This plot of temperature shows what happens to a 75 g sample of steam initially at 1 atm and. Condensing Steam An Exothermic Reaction.
From www.teachoo.com
Which of the reactions is an endothermic reaction? MCQ Science Condensing Steam An Exothermic Reaction In the course of an endothermic process, the system gains heat from the. An exothermic reaction gives off heat energy. Burning wood provides heat through the exothermic chemical reaction of oxygen (o) with cellulose (c 6 h 10 o 5), the major. This plot of temperature shows what happens to a 75 g sample of steam initially at 1 atm. Condensing Steam An Exothermic Reaction.
From byjus.com
Are combination reactions always exothermic Condensing Steam An Exothermic Reaction This typically occurs when water vapor molecules. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Exothermic reactions feel warm or hot or may even be. Exothermic reactions are reactions that release energy into the environment in the form of heat. Burning wood provides heat through the exothermic chemical reaction of. Condensing Steam An Exothermic Reaction.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Condensing Steam An Exothermic Reaction Exothermic reactions are reactions that release energy into the environment in the form of heat. This typically occurs when water vapor molecules. Exothermic reactions feel warm or hot or may even be. This plot of temperature shows what happens to a 75 g sample of steam initially at 1 atm and 200°c as heat is removed at a constant rate:. Condensing Steam An Exothermic Reaction.
From higheducationlearning.com
Is Sublimation Endothermic Or Exothermic Process? » Education Tips Condensing Steam An Exothermic Reaction This plot of temperature shows what happens to a 75 g sample of steam initially at 1 atm and 200°c as heat is removed at a constant rate: Exothermic reactions feel warm or hot or may even be. Condensation is the process by which water vapor turns into liquid water. An exothermic reaction gives off heat energy. A chemical reaction. Condensing Steam An Exothermic Reaction.
From paktechpoint.com
Exothermic Reactions and It's Examples Condensing Steam An Exothermic Reaction Exothermic reactions feel warm or hot or may even be. The reaction produces heat, steam, carbon dioxide (co 2 ), and sulfur dioxide (so 2 ). A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. An exothermic reaction gives off heat energy. C 12 h 22 o 11 (s) + h. Condensing Steam An Exothermic Reaction.
From slideplayer.com
Endothermic & Exothermic Reactions ppt download Condensing Steam An Exothermic Reaction An exothermic reaction gives off heat energy. Exothermic reactions are reactions that release energy into the environment in the form of heat. C 12 h 22 o 11 (s) + h 2 so 4 (aq.) + ½ o 2 (g) → 11 c (s) + 12 h 2 o (l) + co 2 (g) + so 2 (g) + heat. Condensing Steam An Exothermic Reaction.
From www.adda247.com
Exothermic Reaction Examples, Equation, Definition, Meaning Condensing Steam An Exothermic Reaction A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Sulfuric acid reacts with ordinary table sugar (sucrose) and results in an energetic exothermic reaction. Burning wood provides heat through the exothermic chemical reaction of oxygen (o) with cellulose (c 6 h 10 o 5), the major. An exothermic reaction gives off. Condensing Steam An Exothermic Reaction.
From learn.careers360.com
What is exothermic reactions Condensing Steam An Exothermic Reaction Condensation is the process by which water vapor turns into liquid water. An exothermic reaction gives off heat energy. Exothermic reactions feel warm or hot or may even be. Exothermic reactions are reactions that release energy into the environment in the form of heat. A chemical reaction or physical change is endothermic if heat is absorbed by the system from. Condensing Steam An Exothermic Reaction.
From pixellepaper.web.fc2.com
What happens in an exothermic reaction? Condensing Steam An Exothermic Reaction This typically occurs when water vapor molecules. This plot of temperature shows what happens to a 75 g sample of steam initially at 1 atm and 200°c as heat is removed at a constant rate: Sulfuric acid reacts with ordinary table sugar (sucrose) and results in an energetic exothermic reaction. Exothermic reactions feel warm or hot or may even be.. Condensing Steam An Exothermic Reaction.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Condensing Steam An Exothermic Reaction The reaction produces heat, steam, carbon dioxide (co 2 ), and sulfur dioxide (so 2 ). Sulfuric acid reacts with ordinary table sugar (sucrose) and results in an energetic exothermic reaction. In the course of an endothermic process, the system gains heat from the. This typically occurs when water vapor molecules. This plot of temperature shows what happens to a. Condensing Steam An Exothermic Reaction.
From kunduz.com
Exothermic Reaction Definition, Properties, Examples, Exothermic and Condensing Steam An Exothermic Reaction This plot of temperature shows what happens to a 75 g sample of steam initially at 1 atm and 200°c as heat is removed at a constant rate: Sulfuric acid reacts with ordinary table sugar (sucrose) and results in an energetic exothermic reaction. C 12 h 22 o 11 (s) + h 2 so 4 (aq.) + ½ o 2. Condensing Steam An Exothermic Reaction.
From www.chegg.com
Solved Т. Е. pc, CA. IF F pc, Cpc In a backstirred reactor Condensing Steam An Exothermic Reaction Exothermic reactions feel warm or hot or may even be. Burning wood provides heat through the exothermic chemical reaction of oxygen (o) with cellulose (c 6 h 10 o 5), the major. Exothermic reactions are reactions that release energy into the environment in the form of heat. This plot of temperature shows what happens to a 75 g sample of. Condensing Steam An Exothermic Reaction.
From www.numerade.com
SOLVED Identify whether the combustion of propane is exothermic or Condensing Steam An Exothermic Reaction This plot of temperature shows what happens to a 75 g sample of steam initially at 1 atm and 200°c as heat is removed at a constant rate: In the course of an endothermic process, the system gains heat from the. Exothermic reactions feel warm or hot or may even be. A chemical reaction or physical change is exothermic if. Condensing Steam An Exothermic Reaction.
From khatiar1955a.altervista.org
The Difference Between Endothermic and Exothermic Reactions Condensing Steam An Exothermic Reaction Exothermic reactions feel warm or hot or may even be. Condensation is the process by which water vapor turns into liquid water. Sulfuric acid reacts with ordinary table sugar (sucrose) and results in an energetic exothermic reaction. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. In the course of an. Condensing Steam An Exothermic Reaction.
From slideplayer.com
Endothermic and Exothermic Reactions ppt download Condensing Steam An Exothermic Reaction Sulfuric acid reacts with ordinary table sugar (sucrose) and results in an energetic exothermic reaction. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Burning wood provides heat through the exothermic chemical reaction of oxygen (o) with cellulose (c 6 h 10 o 5), the major. Exothermic reactions feel warm or. Condensing Steam An Exothermic Reaction.
From mavink.com
Exothermic Chemical Reaction Examples Condensing Steam An Exothermic Reaction In the course of an endothermic process, the system gains heat from the. This typically occurs when water vapor molecules. The reaction produces heat, steam, carbon dioxide (co 2 ), and sulfur dioxide (so 2 ). C 12 h 22 o 11 (s) + h 2 so 4 (aq.) + ½ o 2 (g) → 11 c (s) + 12. Condensing Steam An Exothermic Reaction.
From www.slideshare.net
Exothermic reactions Condensing Steam An Exothermic Reaction C 12 h 22 o 11 (s) + h 2 so 4 (aq.) + ½ o 2 (g) → 11 c (s) + 12 h 2 o (l) + co 2 (g) + so 2 (g) + heat A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. This typically occurs when. Condensing Steam An Exothermic Reaction.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Condensing Steam An Exothermic Reaction Sulfuric acid reacts with ordinary table sugar (sucrose) and results in an energetic exothermic reaction. Exothermic reactions are reactions that release energy into the environment in the form of heat. This plot of temperature shows what happens to a 75 g sample of steam initially at 1 atm and 200°c as heat is removed at a constant rate: Exothermic reactions. Condensing Steam An Exothermic Reaction.
From eduinput.com
Exothermic ReactionsCharacteristics, Identification, and Examples Condensing Steam An Exothermic Reaction Burning wood provides heat through the exothermic chemical reaction of oxygen (o) with cellulose (c 6 h 10 o 5), the major. An exothermic reaction gives off heat energy. Exothermic reactions are reactions that release energy into the environment in the form of heat. Condensation is the process by which water vapor turns into liquid water. Sulfuric acid reacts with. Condensing Steam An Exothermic Reaction.
From slideplayer.com
Endothermic & Exothermic Reactions ppt download Condensing Steam An Exothermic Reaction C 12 h 22 o 11 (s) + h 2 so 4 (aq.) + ½ o 2 (g) → 11 c (s) + 12 h 2 o (l) + co 2 (g) + so 2 (g) + heat An exothermic reaction gives off heat energy. This plot of temperature shows what happens to a 75 g sample of steam initially. Condensing Steam An Exothermic Reaction.
From scienceinfo.com
Exothermic reactions with Important Examples Condensing Steam An Exothermic Reaction This plot of temperature shows what happens to a 75 g sample of steam initially at 1 atm and 200°c as heat is removed at a constant rate: An exothermic reaction gives off heat energy. C 12 h 22 o 11 (s) + h 2 so 4 (aq.) + ½ o 2 (g) → 11 c (s) + 12 h. Condensing Steam An Exothermic Reaction.
From www.transformationtutoring.com
Which diagram represents the potential energy changes during an Condensing Steam An Exothermic Reaction C 12 h 22 o 11 (s) + h 2 so 4 (aq.) + ½ o 2 (g) → 11 c (s) + 12 h 2 o (l) + co 2 (g) + so 2 (g) + heat Exothermic reactions feel warm or hot or may even be. A chemical reaction or physical change is exothermic if heat is released. Condensing Steam An Exothermic Reaction.
From gideontegarner.blogspot.com
What is Exothermic Reaction GideonteGarner Condensing Steam An Exothermic Reaction Condensation is the process by which water vapor turns into liquid water. Burning wood provides heat through the exothermic chemical reaction of oxygen (o) with cellulose (c 6 h 10 o 5), the major. C 12 h 22 o 11 (s) + h 2 so 4 (aq.) + ½ o 2 (g) → 11 c (s) + 12 h 2. Condensing Steam An Exothermic Reaction.
From online-learning-college.com
Exothermic and endothermic reactions Key differences Condensing Steam An Exothermic Reaction Burning wood provides heat through the exothermic chemical reaction of oxygen (o) with cellulose (c 6 h 10 o 5), the major. Exothermic reactions are reactions that release energy into the environment in the form of heat. The reaction produces heat, steam, carbon dioxide (co 2 ), and sulfur dioxide (so 2 ). Condensation is the process by which water. Condensing Steam An Exothermic Reaction.
From sciencenotes.org
Exothermic Reactions Definition and Examples Condensing Steam An Exothermic Reaction In the course of an endothermic process, the system gains heat from the. This plot of temperature shows what happens to a 75 g sample of steam initially at 1 atm and 200°c as heat is removed at a constant rate: An exothermic reaction gives off heat energy. The reaction produces heat, steam, carbon dioxide (co 2 ), and sulfur. Condensing Steam An Exothermic Reaction.