Pmda Medical Device Database . there are two regulatory authorities responsible for regulation of medical devices in japan: | pharmaceuticals and medical devices agency. [2022/06/14] <md>3 japanese medical device nomenclatures (jmdn) are established and 1 is revised. clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs, medical. safety alert & recalls/ review reports/ package inserts etc. [2024/08/15] <md>information about standards is updated. The ministry of health, labour. — what's new. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. development of drugs, medical devices, regenerative medicines and in vitro diagnostics
from www.frontiersin.org
safety alert & recalls/ review reports/ package inserts etc. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. development of drugs, medical devices, regenerative medicines and in vitro diagnostics | pharmaceuticals and medical devices agency. [2024/08/15] <md>information about standards is updated. — what's new. clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs, medical. The ministry of health, labour. there are two regulatory authorities responsible for regulation of medical devices in japan: [2022/06/14] <md>3 japanese medical device nomenclatures (jmdn) are established and 1 is revised.
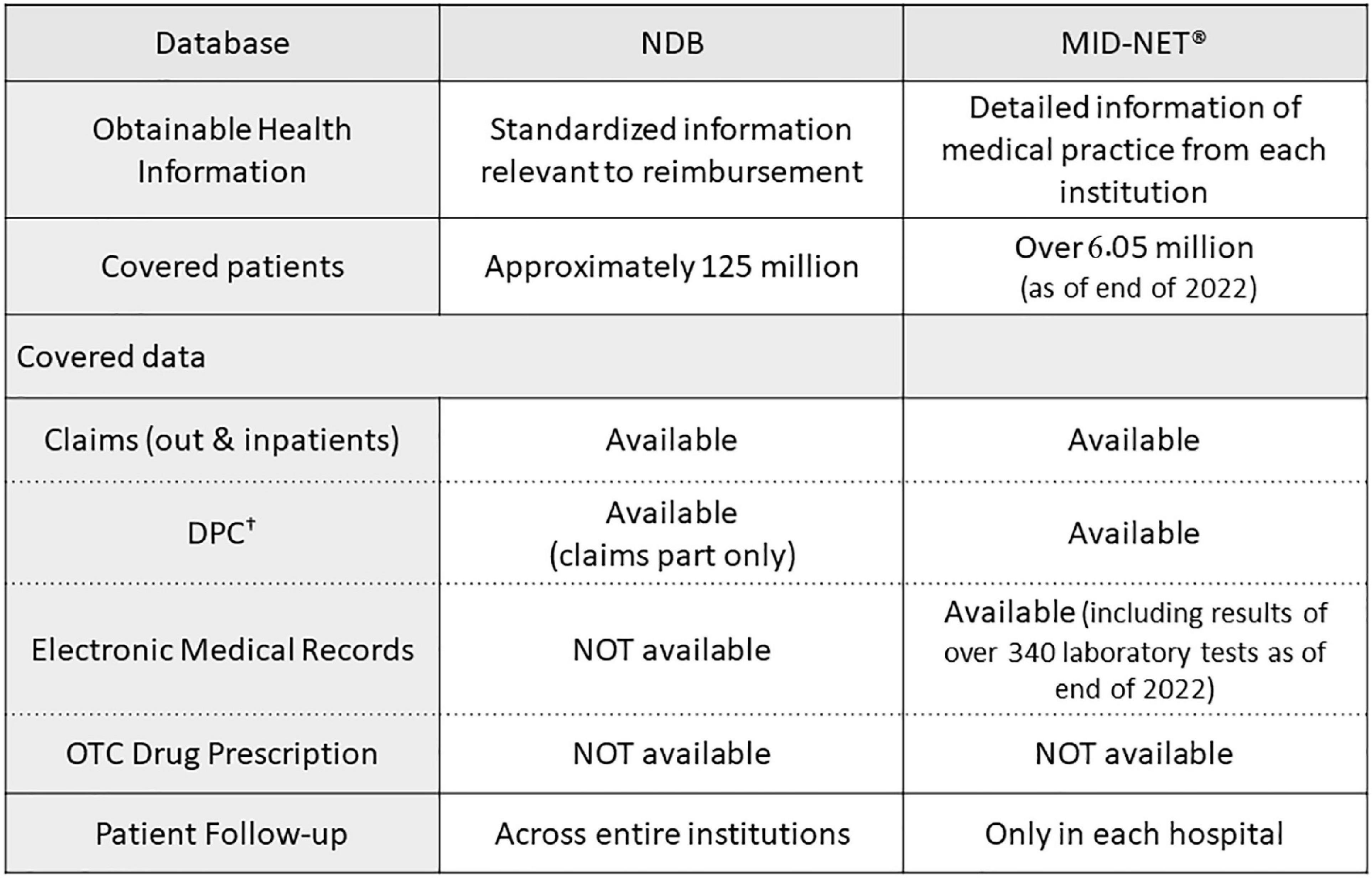
Frontiers Use of National Database of Health Insurance Claims and
Pmda Medical Device Database [2024/08/15] <md>information about standards is updated. The ministry of health, labour. | pharmaceuticals and medical devices agency. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. [2022/06/14] <md>3 japanese medical device nomenclatures (jmdn) are established and 1 is revised. [2024/08/15] <md>information about standards is updated. — what's new. development of drugs, medical devices, regenerative medicines and in vitro diagnostics there are two regulatory authorities responsible for regulation of medical devices in japan: clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs, medical. safety alert & recalls/ review reports/ package inserts etc.
From www.frontiersin.org
Frontiers Use of National Database of Health Insurance Claims and Pmda Medical Device Database [2024/08/15] <md>information about standards is updated. | pharmaceuticals and medical devices agency. safety alert & recalls/ review reports/ package inserts etc. there are two regulatory authorities responsible for regulation of medical devices in japan: development of drugs, medical devices, regenerative medicines and in vitro diagnostics — what's new. The ministry of health, labour. development of. Pmda Medical Device Database.
From www.pmda.go.jp
PMDAATC GMP Inspection Seminar 2016 Pharmaceuticals and Medical Pmda Medical Device Database — what's new. development of drugs, medical devices, regenerative medicines and in vitro diagnostics development of drugs, medical devices, regenerative medicines and in vitro diagnostics. clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs, medical. [2022/06/14] <md>3 japanese medical device nomenclatures (jmdn) are established and 1 is revised. The ministry of health,. Pmda Medical Device Database.
From www.youtube.com
(Medical Device) QMS and Safety Measures PMDAATC Elearning YouTube Pmda Medical Device Database clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs, medical. | pharmaceuticals and medical devices agency. development of drugs, medical devices, regenerative medicines and in vitro diagnostics The ministry of health, labour. safety alert & recalls/ review reports/ package inserts etc. [2024/08/15] <md>information about standards is updated. — what's new. development of. Pmda Medical Device Database.
From docs.oracle.com
Configuration updates in Argus Console Pmda Medical Device Database safety alert & recalls/ review reports/ package inserts etc. development of drugs, medical devices, regenerative medicines and in vitro diagnostics clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs, medical. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. [2022/06/14] <md>3 japanese medical device nomenclatures (jmdn) are established and 1. Pmda Medical Device Database.
From www.scribd.com
PMDA MHLW Japan Regulatory Update IMDRF Meet PDF Medical Device Pmda Medical Device Database development of drugs, medical devices, regenerative medicines and in vitro diagnostics. clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs, medical. [2022/06/14] <md>3 japanese medical device nomenclatures (jmdn) are established and 1 is revised. development of drugs, medical devices, regenerative medicines and in vitro diagnostics [2024/08/15] <md>information about standards is updated. The ministry. Pmda Medical Device Database.
From www.youtube.com
일본 후생노동성 PMDA 화장품 등록 절차(Cosmetic Registration By Pharmaceuticals and Pmda Medical Device Database | pharmaceuticals and medical devices agency. [2024/08/15] <md>information about standards is updated. — what's new. safety alert & recalls/ review reports/ package inserts etc. The ministry of health, labour. clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs, medical. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. development of. Pmda Medical Device Database.
From www.pmda.go.jp
Report of the PMDAATC MRCT Seminar 2017 Pharmaceuticals and Medical Pmda Medical Device Database — what's new. The ministry of health, labour. there are two regulatory authorities responsible for regulation of medical devices in japan: | pharmaceuticals and medical devices agency. [2024/08/15] <md>information about standards is updated. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs,. Pmda Medical Device Database.
From www.researchgate.net
Initiatives of FDA and MHLW/PMDA for Promoting Regulatory Science Pmda Medical Device Database — what's new. development of drugs, medical devices, regenerative medicines and in vitro diagnostics there are two regulatory authorities responsible for regulation of medical devices in japan: [2024/08/15] <md>information about standards is updated. | pharmaceuticals and medical devices agency. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. The ministry of health, labour. . Pmda Medical Device Database.
From patientengagement.synapseconnect.org
PMDA Pharmaceuticals and Medical Devices Agency Pmda Medical Device Database development of drugs, medical devices, regenerative medicines and in vitro diagnostics clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs, medical. safety alert & recalls/ review reports/ package inserts etc. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. [2022/06/14] <md>3 japanese medical device nomenclatures (jmdn) are established and 1. Pmda Medical Device Database.
From www.pmda.go.jp
Outline of Postmarketing Safety Measures Pharmaceuticals and Medical Pmda Medical Device Database safety alert & recalls/ review reports/ package inserts etc. clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs, medical. The ministry of health, labour. [2022/06/14] <md>3 japanese medical device nomenclatures (jmdn) are established and 1 is revised. development of drugs, medical devices, regenerative medicines and in vitro diagnostics — what's new. |. Pmda Medical Device Database.
From www.pmda.go.jp
Reviews Pharmaceuticals and Medical Devices Agency Pmda Medical Device Database safety alert & recalls/ review reports/ package inserts etc. The ministry of health, labour. — what's new. development of drugs, medical devices, regenerative medicines and in vitro diagnostics [2024/08/15] <md>information about standards is updated. | pharmaceuticals and medical devices agency. clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs, medical. [2022/06/14] <md>3. Pmda Medical Device Database.
From www.youtube.com
(Medical Device) Review of Medical Devices PMDAATC Elearning YouTube Pmda Medical Device Database The ministry of health, labour. [2024/08/15] <md>information about standards is updated. development of drugs, medical devices, regenerative medicines and in vitro diagnostics — what's new. there are two regulatory authorities responsible for regulation of medical devices in japan: | pharmaceuticals and medical devices agency. safety alert & recalls/ review reports/ package inserts etc. clinical trials,. Pmda Medical Device Database.
From www.pmda.go.jp
New Drug Review with Electronic Data Pharmaceuticals and Medical Pmda Medical Device Database The ministry of health, labour. [2022/06/14] <md>3 japanese medical device nomenclatures (jmdn) are established and 1 is revised. [2024/08/15] <md>information about standards is updated. development of drugs, medical devices, regenerative medicines and in vitro diagnostics | pharmaceuticals and medical devices agency. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. there are two regulatory. Pmda Medical Device Database.
From patientengagement.synapseconnect.org
Pharmaceuticals and Medical Devices Agency (PMDA) Pmda Medical Device Database | pharmaceuticals and medical devices agency. safety alert & recalls/ review reports/ package inserts etc. The ministry of health, labour. there are two regulatory authorities responsible for regulation of medical devices in japan: [2024/08/15] <md>information about standards is updated. — what's new. [2022/06/14] <md>3 japanese medical device nomenclatures (jmdn) are established and 1 is revised. . Pmda Medical Device Database.
From mindmachineco.com
Medical Device Classifications FDA vs EMA vs MDD vs PMDA Pmda Medical Device Database clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs, medical. safety alert & recalls/ review reports/ package inserts etc. development of drugs, medical devices, regenerative medicines and in vitro diagnostics [2022/06/14] <md>3 japanese medical device nomenclatures (jmdn) are established and 1 is revised. | pharmaceuticals and medical devices agency. there are two. Pmda Medical Device Database.
From globalregulatorypartners.com
PMDA Approach to Artificial Intelligence (AI) based Medical Devices Pmda Medical Device Database — what's new. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. The ministry of health, labour. | pharmaceuticals and medical devices agency. clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs, medical. development of drugs, medical devices, regenerative medicines and in vitro diagnostics [2022/06/14] <md>3 japanese medical device nomenclatures. Pmda Medical Device Database.
From www.pmda.go.jp
Report of the PMDAATC Medical Devices Seminar 2018 Pharmaceuticals Pmda Medical Device Database — what's new. safety alert & recalls/ review reports/ package inserts etc. there are two regulatory authorities responsible for regulation of medical devices in japan: The ministry of health, labour. clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs, medical. | pharmaceuticals and medical devices agency. [2024/08/15] <md>information about standards is updated. . Pmda Medical Device Database.
From medicaldevices.freyrsolutions.com
Medical device registration Japan, PMDA, MHLW, PMDA Medical Device Pmda Medical Device Database The ministry of health, labour. safety alert & recalls/ review reports/ package inserts etc. there are two regulatory authorities responsible for regulation of medical devices in japan: | pharmaceuticals and medical devices agency. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. [2022/06/14] <md>3 japanese medical device nomenclatures (jmdn) are established and 1 is. Pmda Medical Device Database.
From www.youtube.com
(Medical Device) Third Party Certification PMDAATC Learning Videos Pmda Medical Device Database development of drugs, medical devices, regenerative medicines and in vitro diagnostics. — what's new. safety alert & recalls/ review reports/ package inserts etc. there are two regulatory authorities responsible for regulation of medical devices in japan: | pharmaceuticals and medical devices agency. The ministry of health, labour. clinical trials, reviews, consultations, compliance assessments, and inspections. Pmda Medical Device Database.
From www.pmda.go.jp
History Pharmaceuticals and Medical Devices Agency Pmda Medical Device Database development of drugs, medical devices, regenerative medicines and in vitro diagnostics [2024/08/15] <md>information about standards is updated. safety alert & recalls/ review reports/ package inserts etc. [2022/06/14] <md>3 japanese medical device nomenclatures (jmdn) are established and 1 is revised. The ministry of health, labour. — what's new. | pharmaceuticals and medical devices agency. development of. Pmda Medical Device Database.
From www.cphi-online.com
Accreditation Certification of Foreign Drug Manufacturer from Japan PMDA Pmda Medical Device Database clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs, medical. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. safety alert & recalls/ review reports/ package inserts etc. development of drugs, medical devices, regenerative medicines and in vitro diagnostics | pharmaceuticals and medical devices agency. [2022/06/14] <md>3 japanese medical device. Pmda Medical Device Database.
From htlbiotech.com
Products HTL Biotechnology Pmda Medical Device Database — what's new. [2024/08/15] <md>information about standards is updated. The ministry of health, labour. | pharmaceuticals and medical devices agency. development of drugs, medical devices, regenerative medicines and in vitro diagnostics safety alert & recalls/ review reports/ package inserts etc. clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs, medical. there are. Pmda Medical Device Database.
From docs.oracle.com
Device Classification Pmda Medical Device Database development of drugs, medical devices, regenerative medicines and in vitro diagnostics. The ministry of health, labour. clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs, medical. [2024/08/15] <md>information about standards is updated. development of drugs, medical devices, regenerative medicines and in vitro diagnostics — what's new. [2022/06/14] <md>3 japanese medical device nomenclatures. Pmda Medical Device Database.
From www.slideshare.net
Japan PMDA Medical Device Regulatory Approval Process Pmda Medical Device Database | pharmaceuticals and medical devices agency. The ministry of health, labour. — what's new. there are two regulatory authorities responsible for regulation of medical devices in japan: development of drugs, medical devices, regenerative medicines and in vitro diagnostics safety alert & recalls/ review reports/ package inserts etc. development of drugs, medical devices, regenerative medicines and. Pmda Medical Device Database.
From www.apec.org
Medical Devices CoE by PMDA Pmda Medical Device Database [2022/06/14] <md>3 japanese medical device nomenclatures (jmdn) are established and 1 is revised. development of drugs, medical devices, regenerative medicines and in vitro diagnostics development of drugs, medical devices, regenerative medicines and in vitro diagnostics. The ministry of health, labour. — what's new. | pharmaceuticals and medical devices agency. clinical trials, reviews, consultations, compliance assessments,. Pmda Medical Device Database.
From www.pmda.go.jp
Master File System Pharmaceuticals and Medical Devices Agency Pmda Medical Device Database | pharmaceuticals and medical devices agency. The ministry of health, labour. there are two regulatory authorities responsible for regulation of medical devices in japan: development of drugs, medical devices, regenerative medicines and in vitro diagnostics — what's new. clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs, medical. development of drugs, medical. Pmda Medical Device Database.
From www.pmda.go.jp
Reviews Pharmaceuticals and Medical Devices Agency Pmda Medical Device Database development of drugs, medical devices, regenerative medicines and in vitro diagnostics development of drugs, medical devices, regenerative medicines and in vitro diagnostics. clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs, medical. The ministry of health, labour. safety alert & recalls/ review reports/ package inserts etc. [2024/08/15] <md>information about standards is updated. . Pmda Medical Device Database.
From emmainternational.com
FDA Medical Device Databases Backend Platforms Pmda Medical Device Database — what's new. safety alert & recalls/ review reports/ package inserts etc. clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs, medical. development of drugs, medical devices, regenerative medicines and in vitro diagnostics there are two regulatory authorities responsible for regulation of medical devices in japan: [2024/08/15] <md>information about standards is updated.. Pmda Medical Device Database.
From www.youtube.com
(Medical Device) Review of IVDs PMDAATC Elearning YouTube Pmda Medical Device Database there are two regulatory authorities responsible for regulation of medical devices in japan: — what's new. development of drugs, medical devices, regenerative medicines and in vitro diagnostics [2022/06/14] <md>3 japanese medical device nomenclatures (jmdn) are established and 1 is revised. | pharmaceuticals and medical devices agency. clinical trials, reviews, consultations, compliance assessments, and inspections concerning. Pmda Medical Device Database.
From www.slideserve.com
PPT Pharmacovigilance in Japan. - Overview & Specific drug safety Pmda Medical Device Database there are two regulatory authorities responsible for regulation of medical devices in japan: [2024/08/15] <md>information about standards is updated. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. The ministry of health, labour. development of drugs, medical devices, regenerative medicines and in vitro diagnostics safety alert & recalls/ review reports/ package inserts etc. . Pmda Medical Device Database.
From cardiacvascularnews.com
Corindus Announces Pharmaceutical and Medical Device Agency (PMDA Pmda Medical Device Database | pharmaceuticals and medical devices agency. safety alert & recalls/ review reports/ package inserts etc. [2024/08/15] <md>information about standards is updated. clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs, medical. there are two regulatory authorities responsible for regulation of medical devices in japan: — what's new. development of drugs, medical devices,. Pmda Medical Device Database.
From www.youtube.com
How to use the International Medical Device Database Implant FIles Pmda Medical Device Database [2022/06/14] <md>3 japanese medical device nomenclatures (jmdn) are established and 1 is revised. development of drugs, medical devices, regenerative medicines and in vitro diagnostics The ministry of health, labour. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. [2024/08/15] <md>information about standards is updated. there are two regulatory authorities responsible for regulation of medical. Pmda Medical Device Database.
From desertplatforms.com
FDA Medical Device Databases Desert Platforms Pmda Medical Device Database [2022/06/14] <md>3 japanese medical device nomenclatures (jmdn) are established and 1 is revised. clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs, medical. development of drugs, medical devices, regenerative medicines and in vitro diagnostics — what's new. safety alert & recalls/ review reports/ package inserts etc. [2024/08/15] <md>information about standards is updated.. Pmda Medical Device Database.
From globalregulatorypartners.com
Japan's PMDA Global Regulatory Partners, Inc. Pmda Medical Device Database | pharmaceuticals and medical devices agency. The ministry of health, labour. there are two regulatory authorities responsible for regulation of medical devices in japan: safety alert & recalls/ review reports/ package inserts etc. [2024/08/15] <md>information about standards is updated. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. clinical trials, reviews, consultations, compliance assessments,. Pmda Medical Device Database.
From desertplatforms.com
FDA Medical Device Databases Desert Platforms Pmda Medical Device Database development of drugs, medical devices, regenerative medicines and in vitro diagnostics [2024/08/15] <md>information about standards is updated. safety alert & recalls/ review reports/ package inserts etc. clinical trials, reviews, consultations, compliance assessments, and inspections concerning applications for drugs, medical. [2022/06/14] <md>3 japanese medical device nomenclatures (jmdn) are established and 1 is revised. The ministry of health,. Pmda Medical Device Database.