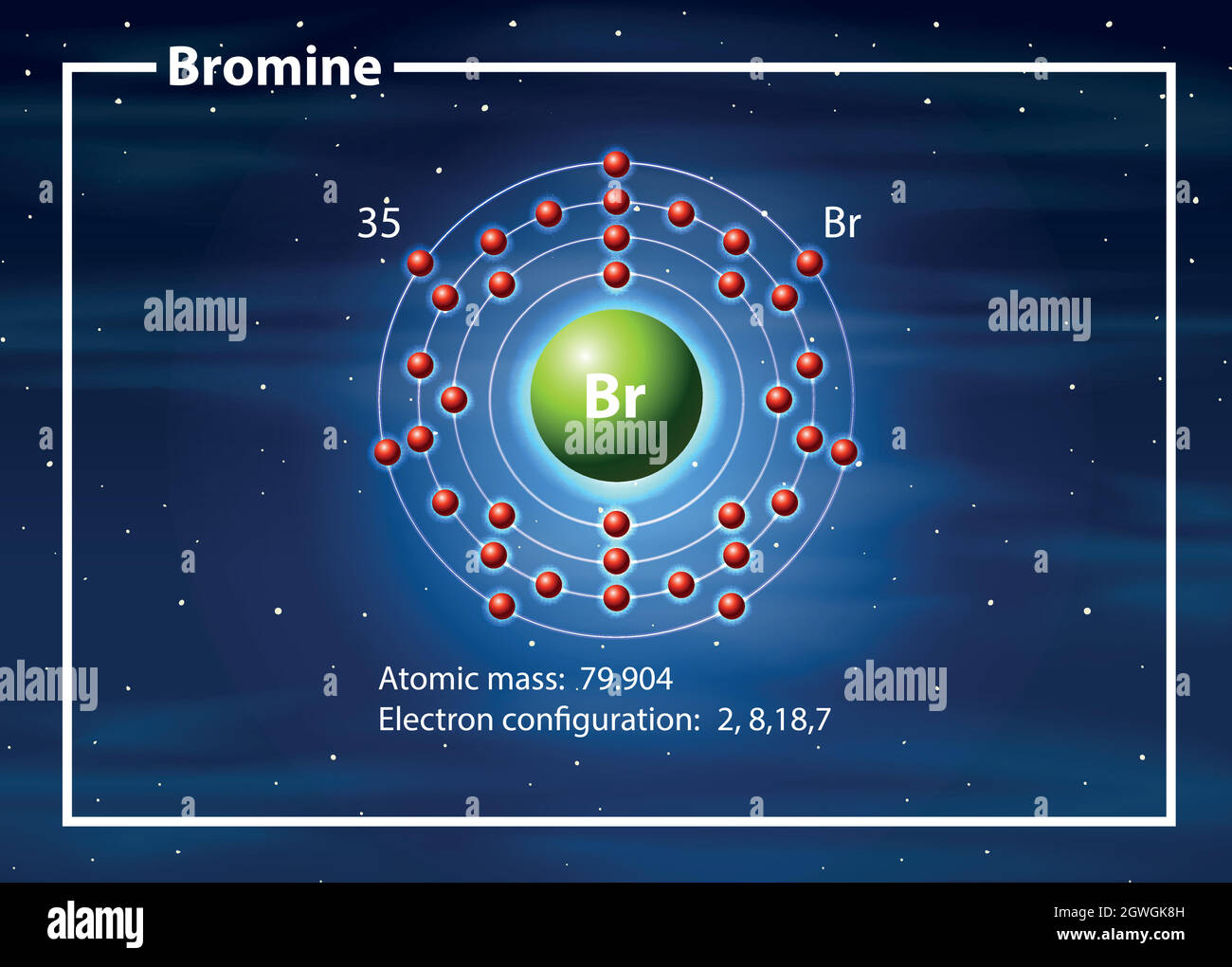
Bromine Stable Ion . At room temperature and pressure, it is one of the few liquid elements. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Bromine is a halogen element with atomic number 35 and element symbol br. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. This electric charge generated on the ion is. Bromine is known for its brown. 93 rows ionic charge:
from www.alamy.com
Bromine is a halogen element with atomic number 35 and element symbol br. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. At room temperature and pressure, it is one of the few liquid elements. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Bromine is known for its brown. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. This electric charge generated on the ion is. 93 rows ionic charge:
Chemist atom of Bromine diagram Stock Vector Image & Art Alamy
Bromine Stable Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). At room temperature and pressure, it is one of the few liquid elements. This electric charge generated on the ion is. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Bromine is a halogen element with atomic number 35 and element symbol br. Bromine is known for its brown. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. 93 rows ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed).
From www.slideserve.com
PPT Chemistry Unit 6 PowerPoint Presentation, free download ID6652385 Bromine Stable Ion At room temperature and pressure, it is one of the few liquid elements. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. 93 rows ionic charge: The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. This electric charge generated on the. Bromine Stable Ion.
From www.researchgate.net
Patterns of the molecular ion with multiple chlorine or bromine atoms.... Download Scientific Bromine Stable Ion This electric charge generated on the ion is. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Bromine is the only nonmetallic element that is liquid at ordinary temperatures. The first ionization energy of bromine is high, and compounds containing bromine. Bromine Stable Ion.
From www.slideserve.com
PPT Chemistry Unit 2 PowerPoint Presentation, free download ID3668529 Bromine Stable Ion At room temperature and pressure, it is one of the few liquid elements. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. This electric charge generated on the ion is. Sources, facts,. Bromine Stable Ion.
From www.britannica.com
bromine Properties, Uses, & Facts Britannica Bromine Stable Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Bromine is a halogen element with atomic number 35 and element symbol br. Bromine is known for its brown. At room temperature and pressure, it is one of. Bromine Stable Ion.
From www.numerade.com
SOLVEDThe only chemically stable ion of rubidium is Rb^+. The most stable monoatomic ion of Bromine Stable Ion For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion. Bromine Stable Ion.
From www.sliderbase.com
Mass Spectrometry Presentation Chemistry Bromine Stable Ion Bromine is a halogen element with atomic number 35 and element symbol br. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. At room temperature and pressure, it is one of the few liquid elements. For. Bromine Stable Ion.
From www.numerade.com
An element's most stable ion forms an ionic compound with bromine, having the formula XBr2. If Bromine Stable Ion For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. 93 rows ionic charge: At room temperature and pressure, it is one of the few liquid elements. Bromine is a halogen element with atomic number 35. Bromine Stable Ion.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Vector Image & Art Alamy Bromine Stable Ion For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. At room temperature and pressure, it is one of the few liquid elements. The first ionization energy of bromine is high, and compounds containing bromine in. Bromine Stable Ion.
From www.youtube.com
Ionic Charge for Bromine (Br) YouTube Bromine Stable Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Bromine is a halogen element with atomic number 35 and element symbol br. At room temperature and pressure, it is one of the few liquid elements. The first ionization energy of bromine is high, and compounds containing bromine in positive. Bromine Stable Ion.
From www.youtube.com
BrO2 with Formal Charges YouTube Bromine Stable Ion Bromine is the only nonmetallic element that is liquid at ordinary temperatures. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Bromine is a halogen element with atomic number 35 and element symbol br. At room temperature and pressure, it is one of the few liquid elements. Bromine is. Bromine Stable Ion.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Bromine Stable Ion The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. 93 rows ionic charge: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. This electric charge generated on the ion is. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. For example, the. Bromine Stable Ion.
From www.numerade.com
SOLVED "Draw the mechanism for the bromination reaction.Pyridinium tribromide releases Bromine Stable Ion For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Bromine is known for its brown. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. This electric charge generated on the ion is. Bromine is a halogen element with atomic number 35 and element symbol br. Bromine is the. Bromine Stable Ion.
From www.masterorganicchemistry.com
More On 1,2 and 1,4 Additions To Dienes Master Organic Chemistry Bromine Stable Ion For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Bromine is a halogen element with atomic number 35 and element symbol br. Bromine is known for its brown. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. When the atom loses or gains one or more electrons, the. Bromine Stable Ion.
From www.alamy.com
Chemist atom of Bromine diagram Stock Vector Image & Art Alamy Bromine Stable Ion Bromine is the only nonmetallic element that is liquid at ordinary temperatures. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Bromine is a halogen element with atomic number 35 and element symbol br. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation. Bromine Stable Ion.
From www.youtube.com
Lec11 Addition of chlorine and bromine to Alkenes and the Bromonium Ion YouTube Bromine Stable Ion Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Bromine is a halogen element with atomic number 35 and element symbol br. Bromine is known for its brown. At room temperature and pressure, it is one of the few liquid elements. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers. Bromine Stable Ion.
From www.numerade.com
Bromine is a halogen; one of the elements in the same column of the points periodic table of Bromine Stable Ion 93 rows ionic charge: For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). At room temperature and pressure, it is one of the few liquid elements. Bromine. Bromine Stable Ion.
From www.youtube.com
Lewis Diagrams and Stable Ions of the Periodic Table SNC1P YouTube Bromine Stable Ion For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Bromine is known for its brown. Bromine is a halogen element with atomic number 35 and element symbol br. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is. Bromine Stable Ion.
From www.chegg.com
Solved 20. Which of the following ions is most stable? B. Br Bromine Stable Ion Bromine is known for its brown. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. 93 rows ionic charge: This electric charge generated on the ion is. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,.. Bromine Stable Ion.
From periodictable.me
How Do We Find The Electron Configuration For Bromine Dynamic Periodic Table of Elements and Bromine Stable Ion Bromine is known for its brown. 93 rows ionic charge: The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. This electric charge generated on the ion is. At room temperature and pressure, it is one of the few liquid elements. Bromine is the only. Bromine Stable Ion.
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine Stable Ion This electric charge generated on the ion is. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The first ionization energy of bromine is high, and compounds. Bromine Stable Ion.
From www.schoolmykids.com
Compare Bromine vs Iodine Periodic Table Element Comparison Compare Properties, Structure, Facts Bromine Stable Ion 93 rows ionic charge: This electric charge generated on the ion is. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Sources,. Bromine Stable Ion.
From studiousguy.com
Bromine (Br) Properties & Uses StudiousGuy Bromine Stable Ion At room temperature and pressure, it is one of the few liquid elements. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Bromine is a halogen element with atomic number 35 and element symbol br. 93 rows ionic charge: Sources, facts, uses, scarcity (sri),. Bromine Stable Ion.
From favpng.com
Electron Configuration Bromine Chemical Element Electron Shell Bohr Model, PNG, 558x600px Bromine Stable Ion Bromine is known for its brown. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. For example, the neutral bromine atom, with 35 protons. Bromine Stable Ion.
From www.semanticscholar.org
Figure 1 from Determination of Bromine Stable Isotope Ratios from Saline Solutions by "Wet Bromine Stable Ion The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. 93 rows ionic charge: This electric charge generated on the ion is. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one. Bromine Stable Ion.
From courses.lumenlearning.com
Periodic Variations in Element Properties CHEM 1305 General Chemistry I—Lecture Bromine Stable Ion The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Bromine. Bromine Stable Ion.
From www.youtube.com
Quick video Balancing an oxidation reduction reaction in base [bromine to bromate and bromide Bromine Stable Ion Bromine is the only nonmetallic element that is liquid at ordinary temperatures. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Bromine is known for its brown. At room temperature and pressure, it is one of. Bromine Stable Ion.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Stable Ion The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Bromine is a halogen element with atomic number 35 and element symbol br. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. This electric. Bromine Stable Ion.
From www.semanticscholar.org
Figure 1 from Determination of Bromine Stable Isotope Ratios from Saline Solutions by "Wet Bromine Stable Ion For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Bromine is known for its brown. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Bromine is a halogen element with atomic number 35 and element symbol. Bromine Stable Ion.
From www.youtube.com
BrO3 Lewis Structure How to Draw the Lewis Structure for BrO3 YouTube Bromine Stable Ion The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. At room temperature and pressure, it is one of the few liquid elements. 93 rows ionic charge: For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide. Bromine Stable Ion.
From h-o-m-e.org
Bromide (Br) Ion Charges Explained Bromine Stable Ion At room temperature and pressure, it is one of the few liquid elements. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Bromine is a halogen element with atomic number 35 and element symbol br. 93 rows ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion. Bromine Stable Ion.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine molecule, and (c) a bromide ion Bromine Stable Ion The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. This electric charge generated on the ion is. At room temperature and pressure, it is one of the few liquid elements. Bromine is a halogen element with atomic number 35 and element symbol br. Bromine. Bromine Stable Ion.
From www.youtube.com
How to Draw the Lewis Dot Structure for Br (Bromide ion) YouTube Bromine Stable Ion Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Bromine is known for its brown. At room temperature and pressure, it is one of the few liquid elements. Bromine is a halogen element with atomic number 35 and element symbol br. 93 rows ionic charge: For example, the neutral bromine atom, with 35 protons and 35 electrons,. Bromine Stable Ion.
From valenceelectrons.com
How to Find the Valence Electrons for Bromine (Br)? Bromine Stable Ion This electric charge generated on the ion is. Bromine is known for its brown. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Bromine is the only nonmetallic element that is liquid at ordinary temperatures. At room temperature and pressure, it is one of the few liquid elements. Sources,. Bromine Stable Ion.
From periodictable.me
2000pxElectron_configuration_bromine.svg Dynamic Periodic Table of Elements and Chemistry Bromine Stable Ion Bromine is known for its brown. This electric charge generated on the ion is. 93 rows ionic charge: The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. When the atom loses or gains one or more electrons, the electric charge is generated (and an. Bromine Stable Ion.
From sciencenotes.org
Bromine Facts Atomic Number 35 and Element Symbol Br Bromine Stable Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. For example, the neutral bromine atom, with. Bromine Stable Ion.