Why Does Salt Water Melt Ice . Salt lowers the freezing point of water. But there’s plenty more to it than that, so we consulted the experts. The answer is fresh water, because the water melting off the ice cube sinks in the plain water and rises in the denser salt water. Salt helps melt ice and prevent it. Because salt particles make it harder for water particles to freeze back onto the ice, the ice that is in contact with dissolved salt. Melting is endothermic, so it lowers the temperature. Why does salt melt ice? When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. This phenomenon is called freezing point depression. Salt only helps if there is a little bit. Let’s start with salt’s relationship. When ice melts, water and ice coexist. Salt makes ice colder because the salt prevents melted water from freezing.
from lessonliblandaulets.z21.web.core.windows.net
Salt only helps if there is a little bit. But there’s plenty more to it than that, so we consulted the experts. Let’s start with salt’s relationship. When ice melts, water and ice coexist. Why does salt melt ice? When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. Melting is endothermic, so it lowers the temperature. Salt helps melt ice and prevent it. The answer is fresh water, because the water melting off the ice cube sinks in the plain water and rises in the denser salt water. Salt makes ice colder because the salt prevents melted water from freezing.
Why Does Salt Melt Ice Chemistry
Why Does Salt Water Melt Ice Because salt particles make it harder for water particles to freeze back onto the ice, the ice that is in contact with dissolved salt. Salt helps melt ice and prevent it. Because salt particles make it harder for water particles to freeze back onto the ice, the ice that is in contact with dissolved salt. Salt only helps if there is a little bit. Let’s start with salt’s relationship. The answer is fresh water, because the water melting off the ice cube sinks in the plain water and rises in the denser salt water. Salt makes ice colder because the salt prevents melted water from freezing. Salt lowers the freezing point of water. But there’s plenty more to it than that, so we consulted the experts. This phenomenon is called freezing point depression. Why does salt melt ice? When ice melts, water and ice coexist. Melting is endothermic, so it lowers the temperature. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing.
From waterseer.org
Does Water Softening Salt Melt Ice? Everything You Need to Know Why Does Salt Water Melt Ice Because salt particles make it harder for water particles to freeze back onto the ice, the ice that is in contact with dissolved salt. This phenomenon is called freezing point depression. Let’s start with salt’s relationship. Melting is endothermic, so it lowers the temperature. The answer is fresh water, because the water melting off the ice cube sinks in the. Why Does Salt Water Melt Ice.
From dengarden.com
The Difference Between Rock Salt and Ice Melt Dengarden Why Does Salt Water Melt Ice Let’s start with salt’s relationship. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. Salt only helps if there is a little bit. Because salt particles make it harder for water particles to freeze back onto the ice, the ice that is in contact with dissolved salt. Salt lowers. Why Does Salt Water Melt Ice.
From www.copecompany.com
Why & How Does Salt Melt Ice? Cope Company Salt Why Does Salt Water Melt Ice This phenomenon is called freezing point depression. But there’s plenty more to it than that, so we consulted the experts. Melting is endothermic, so it lowers the temperature. Salt lowers the freezing point of water. Salt helps melt ice and prevent it. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it. Why Does Salt Water Melt Ice.
From giogfvirt.blob.core.windows.net
Why Does Salt Water Melt Ice Slower Than Freshwater at Dwayne Martinez blog Why Does Salt Water Melt Ice Why does salt melt ice? The answer is fresh water, because the water melting off the ice cube sinks in the plain water and rises in the denser salt water. Salt helps melt ice and prevent it. Salt makes ice colder because the salt prevents melted water from freezing. Because salt particles make it harder for water particles to freeze. Why Does Salt Water Melt Ice.
From giogfvirt.blob.core.windows.net
Why Does Salt Water Melt Ice Slower Than Freshwater at Dwayne Martinez blog Why Does Salt Water Melt Ice Salt makes ice colder because the salt prevents melted water from freezing. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. This phenomenon is called freezing point depression. The answer is fresh water, because the water melting off the ice cube sinks in the plain water and rises in. Why Does Salt Water Melt Ice.
From www.worldatlas.com
Why Does Salt Melt Ice? WorldAtlas Why Does Salt Water Melt Ice Salt makes ice colder because the salt prevents melted water from freezing. This phenomenon is called freezing point depression. Salt helps melt ice and prevent it. But there’s plenty more to it than that, so we consulted the experts. The answer is fresh water, because the water melting off the ice cube sinks in the plain water and rises in. Why Does Salt Water Melt Ice.
From www.metoffice.gov.uk
Sea ice in the climate system Met Office Why Does Salt Water Melt Ice This phenomenon is called freezing point depression. But there’s plenty more to it than that, so we consulted the experts. Melting is endothermic, so it lowers the temperature. Salt makes ice colder because the salt prevents melted water from freezing. Salt only helps if there is a little bit. Salt helps melt ice and prevent it. When ice melts, water. Why Does Salt Water Melt Ice.
From studylib.net
How Does Salt Melt ice and make Ice Cream Why Does Salt Water Melt Ice Melting is endothermic, so it lowers the temperature. When ice melts, water and ice coexist. But there’s plenty more to it than that, so we consulted the experts. Salt makes ice colder because the salt prevents melted water from freezing. Because salt particles make it harder for water particles to freeze back onto the ice, the ice that is in. Why Does Salt Water Melt Ice.
From www.youtube.com
Why Does Salt Melt Ice? SCI CODE YouTube Why Does Salt Water Melt Ice Salt makes ice colder because the salt prevents melted water from freezing. Salt helps melt ice and prevent it. The answer is fresh water, because the water melting off the ice cube sinks in the plain water and rises in the denser salt water. When rock salt is mixed in the water, it lowers the freezing point of the solution,. Why Does Salt Water Melt Ice.
From huntingwaterfalls.com
Why Does Salt Melt Ice Faster Than Sugar? SCIENCE EXPLAINED Why Does Salt Water Melt Ice Let’s start with salt’s relationship. Because salt particles make it harder for water particles to freeze back onto the ice, the ice that is in contact with dissolved salt. When ice melts, water and ice coexist. Melting is endothermic, so it lowers the temperature. Salt helps melt ice and prevent it. Salt only helps if there is a little bit.. Why Does Salt Water Melt Ice.
From sealevel.nasa.gov
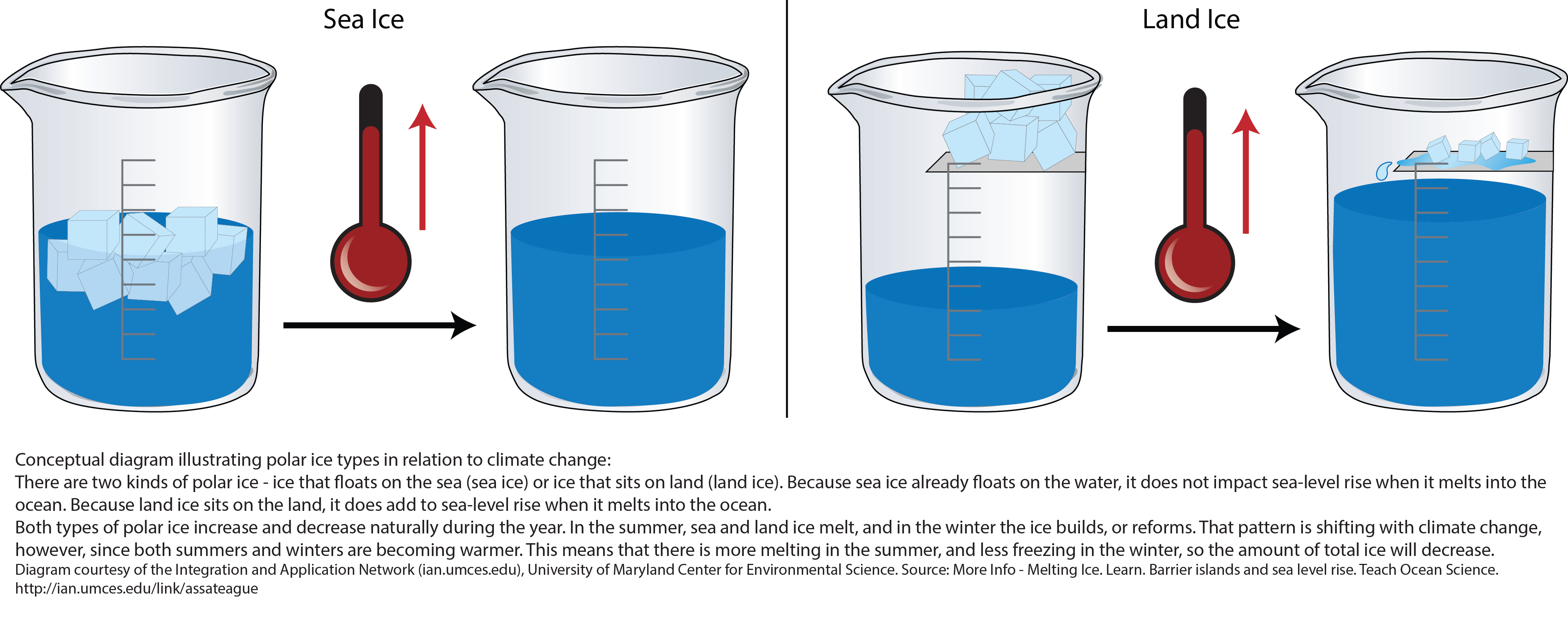
Melting Ocean Ice Affects Sea Level Unlike Ice Cubes in a Glass NASA Sea Level Change Portal Why Does Salt Water Melt Ice When ice melts, water and ice coexist. But there’s plenty more to it than that, so we consulted the experts. This phenomenon is called freezing point depression. Salt lowers the freezing point of water. Because salt particles make it harder for water particles to freeze back onto the ice, the ice that is in contact with dissolved salt. Let’s start. Why Does Salt Water Melt Ice.
From xecogioinhapkhau.com
Does Salt Melt Easily When Heated? Exploring Its Melting Point Why Does Salt Water Melt Ice Salt lowers the freezing point of water. Let’s start with salt’s relationship. Because salt particles make it harder for water particles to freeze back onto the ice, the ice that is in contact with dissolved salt. Why does salt melt ice? When ice melts, water and ice coexist. Melting is endothermic, so it lowers the temperature. Salt only helps if. Why Does Salt Water Melt Ice.
From fox17.com
Behind the Science Why Salt is Used to Melt Ice WZTV Why Does Salt Water Melt Ice When ice melts, water and ice coexist. Salt only helps if there is a little bit. Melting is endothermic, so it lowers the temperature. Salt lowers the freezing point of water. But there’s plenty more to it than that, so we consulted the experts. Why does salt melt ice? Let’s start with salt’s relationship. When rock salt is mixed in. Why Does Salt Water Melt Ice.
From sciencenotes.org
Why Salt Makes Ice Colder How Cold Ice Gets Why Does Salt Water Melt Ice Let’s start with salt’s relationship. Salt helps melt ice and prevent it. The answer is fresh water, because the water melting off the ice cube sinks in the plain water and rises in the denser salt water. Salt only helps if there is a little bit. Why does salt melt ice? Salt lowers the freezing point of water. Because salt. Why Does Salt Water Melt Ice.
From doesitsnowin.com
Why Does Salt Melt Ice? Everything You Need To Know Why Does Salt Water Melt Ice Because salt particles make it harder for water particles to freeze back onto the ice, the ice that is in contact with dissolved salt. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. The answer is fresh water, because the water melting off the ice cube sinks in the. Why Does Salt Water Melt Ice.
From studyzonepatristic.z13.web.core.windows.net
Why Does Adding Salt To Ice Make It Melt Why Does Salt Water Melt Ice Melting is endothermic, so it lowers the temperature. This phenomenon is called freezing point depression. Why does salt melt ice? But there’s plenty more to it than that, so we consulted the experts. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. The answer is fresh water, because the. Why Does Salt Water Melt Ice.
From www.eurekalert.org
How Does Salt Melt Ice? (Video [IMAGE] EurekAlert! Science News Releases Why Does Salt Water Melt Ice But there’s plenty more to it than that, so we consulted the experts. Why does salt melt ice? Salt makes ice colder because the salt prevents melted water from freezing. The answer is fresh water, because the water melting off the ice cube sinks in the plain water and rises in the denser salt water. This phenomenon is called freezing. Why Does Salt Water Melt Ice.
From huntingwaterfalls.com
Why Does Salt Melt Ice? (Simple Science Explained) Why Does Salt Water Melt Ice This phenomenon is called freezing point depression. The answer is fresh water, because the water melting off the ice cube sinks in the plain water and rises in the denser salt water. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. Salt only helps if there is a little. Why Does Salt Water Melt Ice.
From pagingfunmums.com
Melting Ice & Salt Science Experiment Why Does Salt Water Melt Ice Salt lowers the freezing point of water. But there’s plenty more to it than that, so we consulted the experts. Melting is endothermic, so it lowers the temperature. Let’s start with salt’s relationship. Because salt particles make it harder for water particles to freeze back onto the ice, the ice that is in contact with dissolved salt. Salt helps melt. Why Does Salt Water Melt Ice.
From trangtraigarung.com
Why Does Ice Melt Slower In Salt Water? Exploring The Science Why Does Salt Water Melt Ice Let’s start with salt’s relationship. Salt helps melt ice and prevent it. Salt only helps if there is a little bit. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. But there’s plenty more to it than that, so we consulted the experts. This phenomenon is called freezing point. Why Does Salt Water Melt Ice.
From www.thoughtco.com
Why Does Salt Melt Ice? Understanding How It Works Why Does Salt Water Melt Ice Salt helps melt ice and prevent it. When ice melts, water and ice coexist. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. Salt lowers the freezing point of water. Salt only helps if there is a little bit. This phenomenon is called freezing point depression. The answer is. Why Does Salt Water Melt Ice.
From saltsmart.org
How Does Salt Melt Snow and Ice? Salt Smart Collaborative Why Does Salt Water Melt Ice When ice melts, water and ice coexist. Let’s start with salt’s relationship. Salt makes ice colder because the salt prevents melted water from freezing. Salt lowers the freezing point of water. Salt helps melt ice and prevent it. But there’s plenty more to it than that, so we consulted the experts. This phenomenon is called freezing point depression. Melting is. Why Does Salt Water Melt Ice.
From huntingwaterfalls.com
Why Does Salt Make Ice Colder? THE ACTUAL REASON Why Does Salt Water Melt Ice Salt helps melt ice and prevent it. This phenomenon is called freezing point depression. Why does salt melt ice? Salt lowers the freezing point of water. The answer is fresh water, because the water melting off the ice cube sinks in the plain water and rises in the denser salt water. When rock salt is mixed in the water, it. Why Does Salt Water Melt Ice.
From learningnadeausurfier.z21.web.core.windows.net
How Do Salt Melt Ice Why Does Salt Water Melt Ice Salt makes ice colder because the salt prevents melted water from freezing. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. When ice melts, water and ice coexist. Salt helps melt ice and prevent it. The answer is fresh water, because the water melting off the ice cube sinks. Why Does Salt Water Melt Ice.
From materialmcgheepearter.z21.web.core.windows.net
Why Does Ice Melt With Salt Why Does Salt Water Melt Ice Because salt particles make it harder for water particles to freeze back onto the ice, the ice that is in contact with dissolved salt. But there’s plenty more to it than that, so we consulted the experts. The answer is fresh water, because the water melting off the ice cube sinks in the plain water and rises in the denser. Why Does Salt Water Melt Ice.
From giogfvirt.blob.core.windows.net
Why Does Salt Water Melt Ice Slower Than Freshwater at Dwayne Martinez blog Why Does Salt Water Melt Ice Why does salt melt ice? Melting is endothermic, so it lowers the temperature. Salt helps melt ice and prevent it. Salt makes ice colder because the salt prevents melted water from freezing. But there’s plenty more to it than that, so we consulted the experts. Salt lowers the freezing point of water. Salt only helps if there is a little. Why Does Salt Water Melt Ice.
From www.wearegreenbay.com
What melts ice the fastest? Science Course with Ryan Morse Why Does Salt Water Melt Ice Let’s start with salt’s relationship. The answer is fresh water, because the water melting off the ice cube sinks in the plain water and rises in the denser salt water. Why does salt melt ice? Salt lowers the freezing point of water. Salt only helps if there is a little bit. Salt makes ice colder because the salt prevents melted. Why Does Salt Water Melt Ice.
From www.scientificamerican.com
Salt Doesn't Melt IceHere's How It Makes Winter Streets Safer Scientific American Why Does Salt Water Melt Ice When ice melts, water and ice coexist. This phenomenon is called freezing point depression. Salt makes ice colder because the salt prevents melted water from freezing. Let’s start with salt’s relationship. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. Melting is endothermic, so it lowers the temperature. Because. Why Does Salt Water Melt Ice.
From materialmcgheeclinker.z21.web.core.windows.net
How Do Salt Melt Ice Why Does Salt Water Melt Ice The answer is fresh water, because the water melting off the ice cube sinks in the plain water and rises in the denser salt water. Because salt particles make it harder for water particles to freeze back onto the ice, the ice that is in contact with dissolved salt. Salt makes ice colder because the salt prevents melted water from. Why Does Salt Water Melt Ice.
From www.youtube.com
Salt, Sugar or Hot Water Which melts ice faster? YouTube Why Does Salt Water Melt Ice When ice melts, water and ice coexist. The answer is fresh water, because the water melting off the ice cube sinks in the plain water and rises in the denser salt water. Melting is endothermic, so it lowers the temperature. This phenomenon is called freezing point depression. Salt lowers the freezing point of water. Why does salt melt ice? When. Why Does Salt Water Melt Ice.
From dkijlydeeco.blob.core.windows.net
Why Does Salt And Ice Freeze Water at Michelle Fisher blog Why Does Salt Water Melt Ice Why does salt melt ice? The answer is fresh water, because the water melting off the ice cube sinks in the plain water and rises in the denser salt water. But there’s plenty more to it than that, so we consulted the experts. Salt makes ice colder because the salt prevents melted water from freezing. Salt lowers the freezing point. Why Does Salt Water Melt Ice.
From www.branchor.com
Why Does Salt Melt Ice? Understanding the Science and Benefits of Using Salt for Ice Removal Why Does Salt Water Melt Ice The answer is fresh water, because the water melting off the ice cube sinks in the plain water and rises in the denser salt water. Let’s start with salt’s relationship. Salt lowers the freezing point of water. But there’s plenty more to it than that, so we consulted the experts. This phenomenon is called freezing point depression. When ice melts,. Why Does Salt Water Melt Ice.
From www.slideserve.com
PPT Why Do We Add Salt to Ice? PowerPoint Presentation, free download ID6365433 Why Does Salt Water Melt Ice Melting is endothermic, so it lowers the temperature. This phenomenon is called freezing point depression. Salt makes ice colder because the salt prevents melted water from freezing. Because salt particles make it harder for water particles to freeze back onto the ice, the ice that is in contact with dissolved salt. Salt only helps if there is a little bit.. Why Does Salt Water Melt Ice.
From lessonlangdonslurp.z21.web.core.windows.net
What Happens When Salt And Ice Mix Why Does Salt Water Melt Ice Let’s start with salt’s relationship. Why does salt melt ice? Salt only helps if there is a little bit. This phenomenon is called freezing point depression. When ice melts, water and ice coexist. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. Melting is endothermic, so it lowers the. Why Does Salt Water Melt Ice.
From lessonliblandaulets.z21.web.core.windows.net
Why Does Salt Melt Ice Chemistry Why Does Salt Water Melt Ice Salt makes ice colder because the salt prevents melted water from freezing. Salt lowers the freezing point of water. Salt helps melt ice and prevent it. The answer is fresh water, because the water melting off the ice cube sinks in the plain water and rises in the denser salt water. Why does salt melt ice? Because salt particles make. Why Does Salt Water Melt Ice.