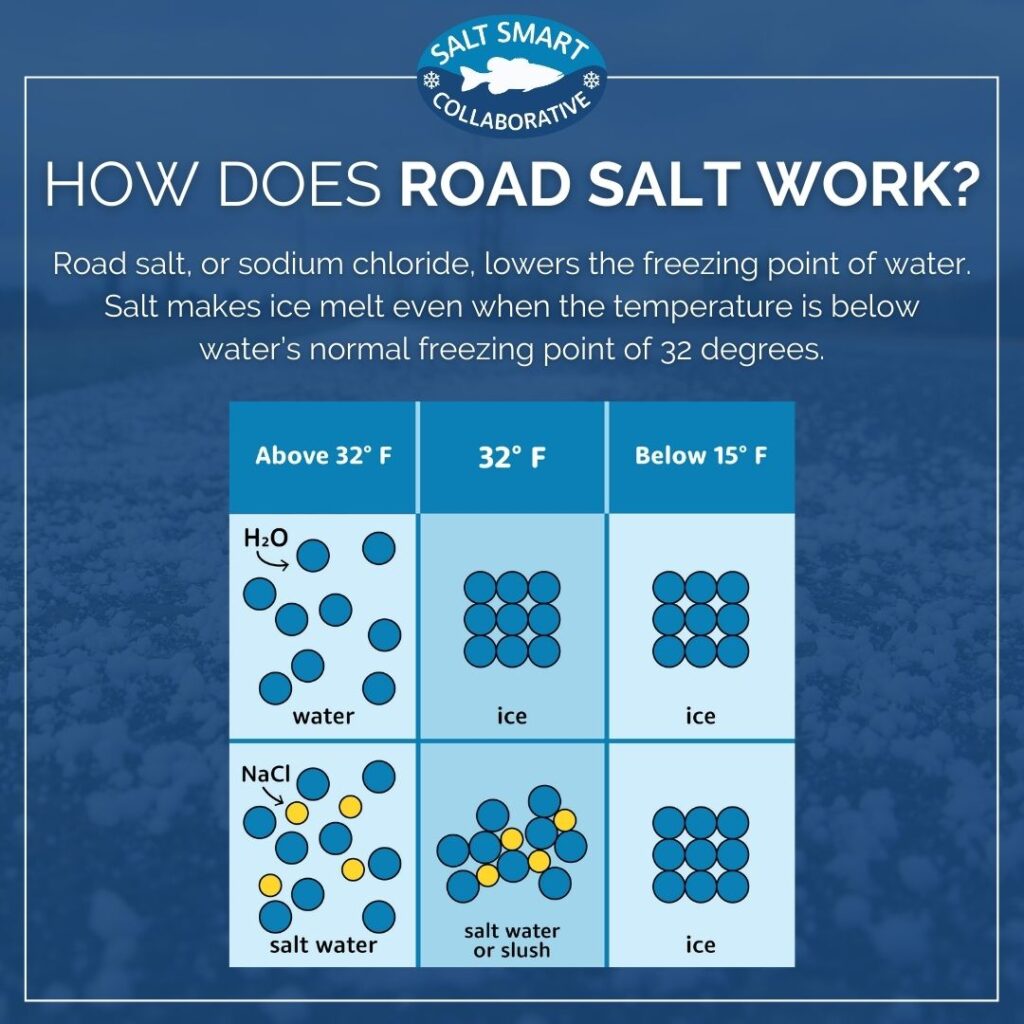
How Does Salt Help To Melt Ice . Salt makes ice colder because the salt prevents melted water from freezing. Melting is endothermic, so it lowers the temperature. It helps to melt the ice by. If any foreign substance is added to the ice like salt, the water molecules can't attach to form ice as quickly, and so the freezing point (or ice formation rate) is lowered, while the melting rate is unaffected. How does salt affect the ice? Salt lowers the freezing point of water. In water, salt is a solute, and it will break into its elements. Salt only helps if there is a little bit of. Why does salt melt ice. But there’s plenty more to it than that, so we consulted the experts. Why does salt melt ice? You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. When the water has reached an equilibrium at 0 degrees, the ice, undisturbed, will remain ice. The main difference is, that in order to melt the ice, you add the salt to the ice itself, where it lowers the melting point and thus (if the. This phenomenon is called freezing point depression.
from dxoaaraqk.blob.core.windows.net
Salt makes ice colder because the salt prevents melted water from freezing. Salt lowers the freezing point of water. Essentially, the salt makes it harder for the water molecules to bond together in their rigid structure. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. So, if you’re using table salt, also known as. The main difference is, that in order to melt the ice, you add the salt to the ice itself, where it lowers the melting point and thus (if the. It helps to melt the ice by. Let’s start with salt’s relationship. But there’s plenty more to it than that, so we consulted the experts. How does salt affect the ice?
What Can I Use To Melt Snow Instead Of Salt at Angela Crim blog
How Does Salt Help To Melt Ice Why does salt melt ice. In water, salt is a solute, and it will break into its elements. It helps to melt the ice by. Salt lowers the freezing point of water. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. Why does salt melt ice. Salt only helps if there is a little bit of. Salt makes ice colder because the salt prevents melted water from freezing. When the water has reached an equilibrium at 0 degrees, the ice, undisturbed, will remain ice. Essentially, the salt makes it harder for the water molecules to bond together in their rigid structure. This phenomenon is called freezing point depression. If any foreign substance is added to the ice like salt, the water molecules can't attach to form ice as quickly, and so the freezing point (or ice formation rate) is lowered, while the melting rate is unaffected. But there’s plenty more to it than that, so we consulted the experts. So, if you’re using table salt, also known as. How does salt affect the ice? The main difference is, that in order to melt the ice, you add the salt to the ice itself, where it lowers the melting point and thus (if the.
From joijbfymp.blob.core.windows.net
Does Hot Or Cold Water Melt Ice Faster at Jenny Carroll blog How Does Salt Help To Melt Ice How does salt affect the ice? Essentially, the salt makes it harder for the water molecules to bond together in their rigid structure. But there’s plenty more to it than that, so we consulted the experts. Melting is endothermic, so it lowers the temperature. Salt only helps if there is a little bit of. Salt lowers the freezing point of. How Does Salt Help To Melt Ice.
From waterseer.org
Does Water Softening Salt Melt Ice? Everything You Need to Know How Does Salt Help To Melt Ice Melting is endothermic, so it lowers the temperature. This phenomenon is called freezing point depression. How does salt affect the ice? Salt only helps if there is a little bit of. Salt makes ice colder because the salt prevents melted water from freezing. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. It helps to. How Does Salt Help To Melt Ice.
From klailpazu.blob.core.windows.net
What Melts Ice Faster Vinegar Or Salt at Herman Hebert blog How Does Salt Help To Melt Ice Why does salt melt ice? Let’s start with salt’s relationship. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. Salt makes ice colder because the salt prevents melted water from freezing. The main difference is, that in order to melt the ice, you add the salt to the ice itself, where it lowers the melting. How Does Salt Help To Melt Ice.
From trangtraigarung.com
Why Does Ice Melt Slower In Salt Water? Exploring The Science How Does Salt Help To Melt Ice This phenomenon is called freezing point depression. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. In water, salt is a solute, and it will break into its elements. Melting is endothermic, so it lowers the temperature. Let’s start with salt’s relationship. If any foreign substance is added to the ice like salt, the water. How Does Salt Help To Melt Ice.
From www.science-sparks.com
Why does salt melt ice? How Does Salt Help To Melt Ice Let’s start with salt’s relationship. In water, salt is a solute, and it will break into its elements. Essentially, the salt makes it harder for the water molecules to bond together in their rigid structure. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. The main difference is, that in order to melt the ice,. How Does Salt Help To Melt Ice.
From frugalfun4boys.com
Melting Ice Science Experiments {Fun!} Frugal Fun For Boys and Girls How Does Salt Help To Melt Ice It helps to melt the ice by. Why does salt melt ice. This phenomenon is called freezing point depression. Melting is endothermic, so it lowers the temperature. But there’s plenty more to it than that, so we consulted the experts. The main difference is, that in order to melt the ice, you add the salt to the ice itself, where. How Does Salt Help To Melt Ice.
From doesitsnowin.com
Why Does Salt Melt Ice? Everything You Need To Know How Does Salt Help To Melt Ice Salt only helps if there is a little bit of. Why does salt melt ice. In water, salt is a solute, and it will break into its elements. Let’s start with salt’s relationship. So, if you’re using table salt, also known as. Melting is endothermic, so it lowers the temperature. But there’s plenty more to it than that, so we. How Does Salt Help To Melt Ice.
From dxofxtrfm.blob.core.windows.net
Rock Salt Vs Table Salt For Melting Ice at Kevin Dewitt blog How Does Salt Help To Melt Ice Why does salt melt ice. But there’s plenty more to it than that, so we consulted the experts. Salt lowers the freezing point of water. Salt only helps if there is a little bit of. Salt makes ice colder because the salt prevents melted water from freezing. The main difference is, that in order to melt the ice, you add. How Does Salt Help To Melt Ice.
From loennxpag.blob.core.windows.net
Why Does Adding Salt To Ice Make It Melt at Charles Pearson blog How Does Salt Help To Melt Ice Essentially, the salt makes it harder for the water molecules to bond together in their rigid structure. Why does salt melt ice. Let’s start with salt’s relationship. Salt makes ice colder because the salt prevents melted water from freezing. Why does salt melt ice? You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. So, if. How Does Salt Help To Melt Ice.
From www.lowes.com
Rock salt Ice Melt at How Does Salt Help To Melt Ice Let’s start with salt’s relationship. It helps to melt the ice by. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. But there’s plenty more to it than that, so we consulted the experts. The main difference is, that in order to melt the ice, you add the salt to the ice itself, where it. How Does Salt Help To Melt Ice.
From learningfullcomtist.z14.web.core.windows.net
How Does Sugar Melt Ice How Does Salt Help To Melt Ice Why does salt melt ice. Let’s start with salt’s relationship. Melting is endothermic, so it lowers the temperature. But there’s plenty more to it than that, so we consulted the experts. Salt makes ice colder because the salt prevents melted water from freezing. In water, salt is a solute, and it will break into its elements. When the water has. How Does Salt Help To Melt Ice.
From frugalfun4boys.com
Don't Melt the Ice! Science Experiment for Kids Frugal Fun For Boys How Does Salt Help To Melt Ice This phenomenon is called freezing point depression. Let’s start with salt’s relationship. In water, salt is a solute, and it will break into its elements. The main difference is, that in order to melt the ice, you add the salt to the ice itself, where it lowers the melting point and thus (if the. When the water has reached an. How Does Salt Help To Melt Ice.
From www.facebook.com
The Action Lab Salt does NOT melt ICE! 🧊 How Does Salt Help To Melt Ice When the water has reached an equilibrium at 0 degrees, the ice, undisturbed, will remain ice. This phenomenon is called freezing point depression. In water, salt is a solute, and it will break into its elements. Salt lowers the freezing point of water. The main difference is, that in order to melt the ice, you add the salt to the. How Does Salt Help To Melt Ice.
From www.science-sparks.com
Why does salt melt ice? How Does Salt Help To Melt Ice How does salt affect the ice? Let’s start with salt’s relationship. When the water has reached an equilibrium at 0 degrees, the ice, undisturbed, will remain ice. Why does salt melt ice? The main difference is, that in order to melt the ice, you add the salt to the ice itself, where it lowers the melting point and thus (if. How Does Salt Help To Melt Ice.
From thoughtfullysustainable.com
How Does Salt Affect Ice? A Simple Science Experiment Thoughtfully How Does Salt Help To Melt Ice When the water has reached an equilibrium at 0 degrees, the ice, undisturbed, will remain ice. Melting is endothermic, so it lowers the temperature. Essentially, the salt makes it harder for the water molecules to bond together in their rigid structure. If any foreign substance is added to the ice like salt, the water molecules can't attach to form ice. How Does Salt Help To Melt Ice.
From klactqsxm.blob.core.windows.net
What Melts Faster Snow Or Ice at Aurelia Buchanan blog How Does Salt Help To Melt Ice Salt lowers the freezing point of water. So, if you’re using table salt, also known as. If any foreign substance is added to the ice like salt, the water molecules can't attach to form ice as quickly, and so the freezing point (or ice formation rate) is lowered, while the melting rate is unaffected. This phenomenon is called freezing point. How Does Salt Help To Melt Ice.
From www.wearegreenbay.com
What melts ice the fastest? Science Course with Storm Team 5 WFRV How Does Salt Help To Melt Ice Why does salt melt ice. The main difference is, that in order to melt the ice, you add the salt to the ice itself, where it lowers the melting point and thus (if the. Salt lowers the freezing point of water. Salt makes ice colder because the salt prevents melted water from freezing. Why does salt melt ice? In water,. How Does Salt Help To Melt Ice.
From dxoyqkogv.blob.core.windows.net
Why Does Salt Melt Ice Experiment at Pamela Gibbs blog How Does Salt Help To Melt Ice Salt only helps if there is a little bit of. If any foreign substance is added to the ice like salt, the water molecules can't attach to form ice as quickly, and so the freezing point (or ice formation rate) is lowered, while the melting rate is unaffected. Salt lowers the freezing point of water. Let’s start with salt’s relationship.. How Does Salt Help To Melt Ice.
From frugalfun4boys.com
Melting Ice Science Experiments {Fun!} Frugal Fun For Boys and Girls How Does Salt Help To Melt Ice Let’s start with salt’s relationship. How does salt affect the ice? Why does salt melt ice. So, if you’re using table salt, also known as. When the water has reached an equilibrium at 0 degrees, the ice, undisturbed, will remain ice. Essentially, the salt makes it harder for the water molecules to bond together in their rigid structure. This phenomenon. How Does Salt Help To Melt Ice.
From www.wearegreenbay.com
What melts ice the fastest? Science Course with Ryan Morse How Does Salt Help To Melt Ice So, if you’re using table salt, also known as. Why does salt melt ice. Why does salt melt ice? Salt lowers the freezing point of water. Salt only helps if there is a little bit of. Melting is endothermic, so it lowers the temperature. Let’s start with salt’s relationship. If any foreign substance is added to the ice like salt,. How Does Salt Help To Melt Ice.
From huntingwaterfalls.com
Why Does Salt Melt Ice Faster Than Sugar? SCIENCE EXPLAINED How Does Salt Help To Melt Ice Why does salt melt ice? Why does salt melt ice. This phenomenon is called freezing point depression. If any foreign substance is added to the ice like salt, the water molecules can't attach to form ice as quickly, and so the freezing point (or ice formation rate) is lowered, while the melting rate is unaffected. When the water has reached. How Does Salt Help To Melt Ice.
From frugalfun4boys.com
Melting Ice Science Experiments {Fun!} Frugal Fun For Boys and Girls How Does Salt Help To Melt Ice Melting is endothermic, so it lowers the temperature. Essentially, the salt makes it harder for the water molecules to bond together in their rigid structure. In water, salt is a solute, and it will break into its elements. Salt lowers the freezing point of water. It helps to melt the ice by. When the water has reached an equilibrium at. How Does Salt Help To Melt Ice.
From huntingwaterfalls.com
Does Salt Make Ice Last Longer? No, But Also Yes How Does Salt Help To Melt Ice In water, salt is a solute, and it will break into its elements. This phenomenon is called freezing point depression. Salt lowers the freezing point of water. So, if you’re using table salt, also known as. Salt makes ice colder because the salt prevents melted water from freezing. Why does salt melt ice? How does salt affect the ice? When. How Does Salt Help To Melt Ice.
From dxoaaraqk.blob.core.windows.net
What Can I Use To Melt Snow Instead Of Salt at Angela Crim blog How Does Salt Help To Melt Ice How does salt affect the ice? In water, salt is a solute, and it will break into its elements. It helps to melt the ice by. This phenomenon is called freezing point depression. Why does salt melt ice? If any foreign substance is added to the ice like salt, the water molecules can't attach to form ice as quickly, and. How Does Salt Help To Melt Ice.
From www.thoughtco.com
Why Does Salt Melt Ice? Understanding How It Works How Does Salt Help To Melt Ice When the water has reached an equilibrium at 0 degrees, the ice, undisturbed, will remain ice. Let’s start with salt’s relationship. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. The main difference is, that in order to melt the ice, you add the salt to the ice itself, where it lowers the melting point. How Does Salt Help To Melt Ice.
From www.studyladder.com
Experiment What effect does salt have on ice? Studyladder How Does Salt Help To Melt Ice Why does salt melt ice. Salt makes ice colder because the salt prevents melted water from freezing. Why does salt melt ice? Let’s start with salt’s relationship. How does salt affect the ice? It helps to melt the ice by. Salt lowers the freezing point of water. When the water has reached an equilibrium at 0 degrees, the ice, undisturbed,. How Does Salt Help To Melt Ice.
From sealevel.nasa.gov
Melting Ocean Ice Affects Sea Level Unlike Ice Cubes in a Glass How Does Salt Help To Melt Ice Salt lowers the freezing point of water. The main difference is, that in order to melt the ice, you add the salt to the ice itself, where it lowers the melting point and thus (if the. It helps to melt the ice by. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. Why does salt. How Does Salt Help To Melt Ice.
From huntingwaterfalls.com
Why Does Salt Melt Ice Faster Than Sugar? SCIENCE EXPLAINED How Does Salt Help To Melt Ice How does salt affect the ice? Let’s start with salt’s relationship. If any foreign substance is added to the ice like salt, the water molecules can't attach to form ice as quickly, and so the freezing point (or ice formation rate) is lowered, while the melting rate is unaffected. Melting is endothermic, so it lowers the temperature. Salt makes ice. How Does Salt Help To Melt Ice.
From dengarden.com
The Difference Between Rock Salt and Ice Melt Dengarden How Does Salt Help To Melt Ice The main difference is, that in order to melt the ice, you add the salt to the ice itself, where it lowers the melting point and thus (if the. It helps to melt the ice by. Why does salt melt ice? Salt makes ice colder because the salt prevents melted water from freezing. Melting is endothermic, so it lowers the. How Does Salt Help To Melt Ice.
From loennxpag.blob.core.windows.net
Why Does Adding Salt To Ice Make It Melt at Charles Pearson blog How Does Salt Help To Melt Ice Salt lowers the freezing point of water. The main difference is, that in order to melt the ice, you add the salt to the ice itself, where it lowers the melting point and thus (if the. But there’s plenty more to it than that, so we consulted the experts. Why does salt melt ice. Salt only helps if there is. How Does Salt Help To Melt Ice.
From theconversation.com
Salt doesn't melt ice here's how it actually makes winter streets safe How Does Salt Help To Melt Ice In water, salt is a solute, and it will break into its elements. So, if you’re using table salt, also known as. This phenomenon is called freezing point depression. Essentially, the salt makes it harder for the water molecules to bond together in their rigid structure. It helps to melt the ice by. Salt makes ice colder because the salt. How Does Salt Help To Melt Ice.
From www.youtube.com
Why Does Salt Melt Ice? SCI CODE YouTube How Does Salt Help To Melt Ice Salt lowers the freezing point of water. Why does salt melt ice. The main difference is, that in order to melt the ice, you add the salt to the ice itself, where it lowers the melting point and thus (if the. In water, salt is a solute, and it will break into its elements. Salt only helps if there is. How Does Salt Help To Melt Ice.
From www.eurekalert.org
How Does Salt Melt Ice? (Video [IMAGE] EurekAlert! Science News Releases How Does Salt Help To Melt Ice How does salt affect the ice? Essentially, the salt makes it harder for the water molecules to bond together in their rigid structure. Salt only helps if there is a little bit of. Let’s start with salt’s relationship. When the water has reached an equilibrium at 0 degrees, the ice, undisturbed, will remain ice. But there’s plenty more to it. How Does Salt Help To Melt Ice.
From sciencenotes.org
Why Salt Makes Ice Colder How Cold Ice Gets How Does Salt Help To Melt Ice It helps to melt the ice by. Melting is endothermic, so it lowers the temperature. Salt lowers the freezing point of water. If any foreign substance is added to the ice like salt, the water molecules can't attach to form ice as quickly, and so the freezing point (or ice formation rate) is lowered, while the melting rate is unaffected.. How Does Salt Help To Melt Ice.
From frugalfun4boys.com
Melting Ice Science Experiments {Fun!} Frugal Fun For Boys and Girls How Does Salt Help To Melt Ice Salt makes ice colder because the salt prevents melted water from freezing. It helps to melt the ice by. Melting is endothermic, so it lowers the temperature. So, if you’re using table salt, also known as. In water, salt is a solute, and it will break into its elements. Why does salt melt ice? Salt lowers the freezing point of. How Does Salt Help To Melt Ice.