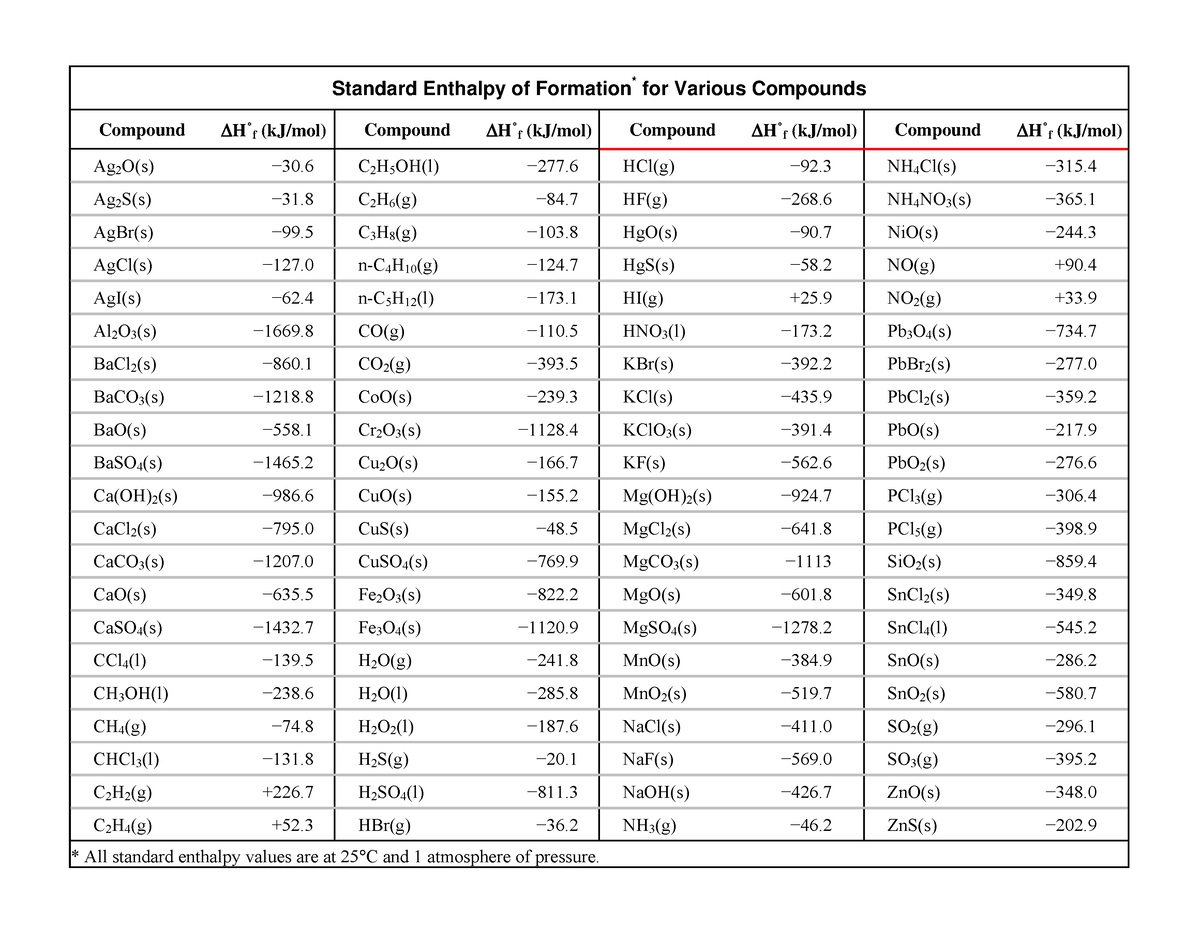
Standard Heat Formation Of Zero . The standard state heat of formation for the elemental form of each atom is zero. The standard enthalpy of formation of oxygen gas is zero. The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation for an element in its standard state is zero!!!! The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. For any element at any particular temperature, we define the standard enthalpy of formation to be zero. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. Elements in their standard state are not formed, they just are.
from www.studocu.com
The standard enthalpy of formation of oxygen gas is zero. For any element at any particular temperature, we define the standard enthalpy of formation to be zero. The standard state heat of formation for the elemental form of each atom is zero. The elemental form of each atom is that with the lowest enthalpy in the standard state. Elements in their standard state are not formed, they just are. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard enthalpy of formation for an element in its standard state is zero!!!! The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for.
Standard Enthalpy of Formation Table Standard Enthalpy of Formation
Standard Heat Formation Of Zero The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation for an element in its standard state is zero!!!! The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard state heat of formation for the elemental form of each atom is zero. The standard enthalpy of formation of oxygen gas is zero. Elements in their standard state are not formed, they just are. For any element at any particular temperature, we define the standard enthalpy of formation to be zero. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for.
From www.studocu.com
Standard Enthalpies of Formation & Standard Entropies kJ J ( mol Standard Heat Formation Of Zero The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under. Standard Heat Formation Of Zero.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation Standard Heat Formation Of Zero The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of oxygen gas is zero. The elemental form of each atom is that with the lowest enthalpy in the standard state. By definition, the standard enthalpy of formation of an element. Standard Heat Formation Of Zero.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Heat Formation Of Zero The standard enthalpy of formation of oxygen gas is zero. The standard state heat of formation for the elemental form of each atom is zero. Elements in their standard state are not formed, they just are. The standard enthalpy of formation for an element in its standard state is zero!!!! The elemental form of each atom is that with the. Standard Heat Formation Of Zero.
From www.slideserve.com
PPT Energetics PowerPoint Presentation ID1204656 Standard Heat Formation Of Zero The standard enthalpy of formation for an element in its standard state is zero!!!! The standard enthalpy of formation of oxygen gas is zero. The elemental form of each atom is that with the lowest enthalpy in the standard state. For any element at any particular temperature, we define the standard enthalpy of formation to be zero. The standard state. Standard Heat Formation Of Zero.
From www.youtube.com
15.1.1 Define Standard State, Enthalpy change of formation and Standard Heat Formation Of Zero The standard enthalpy of formation for an element in its standard state is zero!!!! The standard enthalpy of formation of oxygen gas is zero. The elemental form of each atom is that with the lowest enthalpy in the standard state. Elements in their standard state are not formed, they just are. The standard state heat of formation for the elemental. Standard Heat Formation Of Zero.
From www.slideserve.com
PPT STANDARD HEAT OF FORMATION ΔH 0 f or ΔH θ f PowerPoint Standard Heat Formation Of Zero For any element at any particular temperature, we define the standard enthalpy of formation to be zero. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard enthalpy of formation is a measure of the energy released or consumed. Standard Heat Formation Of Zero.
From www.chegg.com
Solved Calculate the Standard Enthalpy of Formation of NOCl Standard Heat Formation Of Zero The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard enthalpy of formation of oxygen gas is zero. The standard enthalpy of formation. Standard Heat Formation Of Zero.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Heat Formation Of Zero The standard state heat of formation for the elemental form of each atom is zero. Elements in their standard state are not formed, they just are. The standard enthalpy of formation of oxygen gas is zero. For any element at any particular temperature, we define the standard enthalpy of formation to be zero. The standard enthalpy of formation, also known. Standard Heat Formation Of Zero.
From www.slideserve.com
PPT THERMOCHEMISTRY PowerPoint Presentation, free download ID5072129 Standard Heat Formation Of Zero The standard state heat of formation for the elemental form of each atom is zero. Elements in their standard state are not formed, they just are. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard enthalpy of formation. Standard Heat Formation Of Zero.
From www.thoughtco.com
Why the Standard Enthalpy of Formation of O2 Equals Zero Standard Heat Formation Of Zero The standard enthalpy of formation for an element in its standard state is zero!!!! The elemental form of each atom is that with the lowest enthalpy in the standard state. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. Elements in their. Standard Heat Formation Of Zero.
From www.toppr.com
Standard enthalpy of formation is zero for. Standard Heat Formation Of Zero The standard enthalpy of formation of oxygen gas is zero. For any element at any particular temperature, we define the standard enthalpy of formation to be zero. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard state heat. Standard Heat Formation Of Zero.
From www.chegg.com
Solved All of the following have a standard heat of Standard Heat Formation Of Zero Elements in their standard state are not formed, they just are. For any element at any particular temperature, we define the standard enthalpy of formation to be zero. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy of formation. Standard Heat Formation Of Zero.
From www.toppr.com
For which of the following substances, the enthalpy of formation in the Standard Heat Formation Of Zero The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard state heat of formation for the elemental form of each atom is zero. By definition, the standard enthalpy of formation of an element in its most stable form is. Standard Heat Formation Of Zero.
From www.slideshare.net
Tang 03 enthalpy of formation and combustion Standard Heat Formation Of Zero By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. For any element at any particular temperature, we define the standard enthalpy of formation to be zero. Elements in their standard state are not formed, they just are. The standard enthalpy of formation,. Standard Heat Formation Of Zero.
From www.chegg.com
Solved The standard enthalpy of formation of H2O(g) at 298 K Standard Heat Formation Of Zero For any element at any particular temperature, we define the standard enthalpy of formation to be zero. The standard enthalpy of formation of oxygen gas is zero. The standard state heat of formation for the elemental form of each atom is zero. The standard enthalpy of formation for an element in its standard state is zero!!!! The standard enthalpy of. Standard Heat Formation Of Zero.
From www.chegg.com
Solved Which of the substances have a standard heat of Standard Heat Formation Of Zero The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard state heat of formation for the elemental form of each atom is zero. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at.. Standard Heat Formation Of Zero.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Heat Formation Of Zero The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of oxygen gas is zero. For any element at any particular temperature, we define the standard enthalpy of formation to be zero. Elements in their standard state are not formed, they. Standard Heat Formation Of Zero.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Heat Formation Of Zero By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard enthalpy of. Standard Heat Formation Of Zero.
From byjus.com
43. Calculate standard heat of formation of CS2. Given that standard Standard Heat Formation Of Zero The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard state heat of formation for the elemental form of each atom is zero. For any element at any particular temperature, we define the standard enthalpy of formation to be. Standard Heat Formation Of Zero.
From www.slideserve.com
PPT Chapter 15 Standard enthalpy change of a reaction PowerPoint Standard Heat Formation Of Zero The standard state heat of formation for the elemental form of each atom is zero. The standard enthalpy of formation of oxygen gas is zero. For any element at any particular temperature, we define the standard enthalpy of formation to be zero. By definition, the standard enthalpy of formation of an element in its most stable form is equal to. Standard Heat Formation Of Zero.
From www.slideserve.com
PPT Standard Heats of Reaction PowerPoint Presentation, free download Standard Heat Formation Of Zero The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of oxygen gas is zero. The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard enthalpy of formation, also known as the heat. Standard Heat Formation Of Zero.
From www.showme.com
11.4Calculating Heat ChangesStandard Heats of Formation (II) Science Standard Heat Formation Of Zero The standard enthalpy of formation of oxygen gas is zero. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For any element at any particular temperature, we define the standard enthalpy of formation to be zero. The standard enthalpy of formation for an element in. Standard Heat Formation Of Zero.
From gioslhodh.blob.core.windows.net
Standard Enthalpy Of Formation Is Zero For Sulphur at Joseph Morehouse blog Standard Heat Formation Of Zero The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Elements in their standard state are not formed, they just are. For any element at any particular temperature, we define the standard enthalpy of formation to be zero. The standard enthalpy of formation for an element. Standard Heat Formation Of Zero.
From www.slideshare.net
Standard enthalpy of formation Standard Heat Formation Of Zero For any element at any particular temperature, we define the standard enthalpy of formation to be zero. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The elemental form of each atom is that with the lowest enthalpy in the. Standard Heat Formation Of Zero.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Heat Formation Of Zero The standard enthalpy of formation for an element in its standard state is zero!!!! The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Elements in their standard state are not formed, they just are. By definition, the standard enthalpy of formation of an element in. Standard Heat Formation Of Zero.
From zeviernswenson.blogspot.com
Standard Enthalpy of Formation ZeviernSwenson Standard Heat Formation Of Zero The standard enthalpy of formation for an element in its standard state is zero!!!! For any element at any particular temperature, we define the standard enthalpy of formation to be zero. The standard state heat of formation for the elemental form of each atom is zero. The standard enthalpy of formation, also known as the heat of formation, is the. Standard Heat Formation Of Zero.
From kaden-chapter.blogspot.com
Standard Enthalpy Of Formation Table Pdf 30+ Pages Summary [1.8mb Standard Heat Formation Of Zero The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard enthalpy of formation of oxygen gas is zero. For any element at any particular temperature, we define the standard enthalpy of formation to be zero. The standard enthalpy of formation for an element in its standard state is zero!!!! By definition, the. Standard Heat Formation Of Zero.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF Standard Heat Formation Of Zero Elements in their standard state are not formed, they just are. The standard state heat of formation for the elemental form of each atom is zero. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard enthalpy of formation. Standard Heat Formation Of Zero.
From www.slideserve.com
PPT STANDARD HEAT OF FORMATION ΔH 0 f or ΔH θ f PowerPoint Standard Heat Formation Of Zero The standard enthalpy of formation of oxygen gas is zero. The elemental form of each atom is that with the lowest enthalpy in the standard state. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy of formation, also known. Standard Heat Formation Of Zero.
From www.chegg.com
Solved The standard heat of formation, ДНі , is defined as Standard Heat Formation Of Zero The standard state heat of formation for the elemental form of each atom is zero. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. Elements in their standard state are not formed, they just are. For any element at any. Standard Heat Formation Of Zero.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID314871 Standard Heat Formation Of Zero Elements in their standard state are not formed, they just are. The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard state heat of formation for the elemental form of each atom is zero. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1. Standard Heat Formation Of Zero.
From quizzlistreplevies.z13.web.core.windows.net
Formula Of Heat Of Formation Standard Heat Formation Of Zero The standard enthalpy of formation for an element in its standard state is zero!!!! The standard state heat of formation for the elemental form of each atom is zero. The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy. Standard Heat Formation Of Zero.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Heat Formation Of Zero The standard enthalpy of formation for an element in its standard state is zero!!!! The elemental form of each atom is that with the lowest enthalpy in the standard state. Elements in their standard state are not formed, they just are. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero. Standard Heat Formation Of Zero.
From duanerafanan.blogspot.com
DUANE HESS'S LAW Standard Heat Formation Of Zero For any element at any particular temperature, we define the standard enthalpy of formation to be zero. Elements in their standard state are not formed, they just are. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. By definition, the. Standard Heat Formation Of Zero.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Heat Formation Of Zero The standard enthalpy of formation for an element in its standard state is zero!!!! Elements in their standard state are not formed, they just are. The standard state heat of formation for the elemental form of each atom is zero. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under. Standard Heat Formation Of Zero.