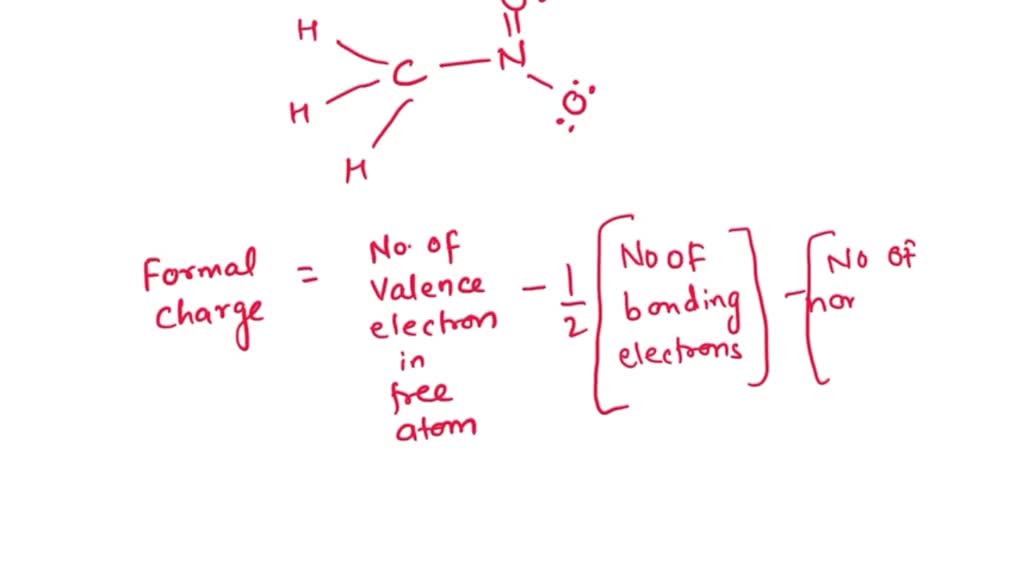
Nitrogen In No2 Formal Charge . Here nitrogen is the free atom and the number of valence. How to calculate formal charge. N = o.around each atom from your left to right there are 9, 7, and 8 electrons respectively leading to formal charges of −1, 0, and 0. If it has four bonds (and no lone pair), it has a formal. Formal charge on nitrogen atom: To calculate the formal charge of an atom, we start by: 3 for boron, 4 for carbon, 5 for nitrogen, and so on). Evaluating the number of valence electrons (ve) the neutral atom has (e.g. So now let’s calculate the formal charge on each individual atom present in no2. Continuing with the nitrogen, we observe that in (a) the nitrogen atom shares three bonding pairs and has one lone pair and has a total of 5 valence. In the left resonance structure, all the atoms have zero formal charge, while on the right structure, the nitrogen has a +1 formal charge, and the. If a nitrogen has three bonds and a lone pair, it has a formal charge of zero.
from www.numerade.com
Formal charge on nitrogen atom: Here nitrogen is the free atom and the number of valence. To calculate the formal charge of an atom, we start by: N = o.around each atom from your left to right there are 9, 7, and 8 electrons respectively leading to formal charges of −1, 0, and 0. If it has four bonds (and no lone pair), it has a formal. In the left resonance structure, all the atoms have zero formal charge, while on the right structure, the nitrogen has a +1 formal charge, and the. If a nitrogen has three bonds and a lone pair, it has a formal charge of zero. How to calculate formal charge. Continuing with the nitrogen, we observe that in (a) the nitrogen atom shares three bonding pairs and has one lone pair and has a total of 5 valence. So now let’s calculate the formal charge on each individual atom present in no2.
SOLVED Calculate the formal charge on nitrogen in CH3NO2 (Hint Lewis structure) 0 1
Nitrogen In No2 Formal Charge How to calculate formal charge. Continuing with the nitrogen, we observe that in (a) the nitrogen atom shares three bonding pairs and has one lone pair and has a total of 5 valence. Formal charge on nitrogen atom: N = o.around each atom from your left to right there are 9, 7, and 8 electrons respectively leading to formal charges of −1, 0, and 0. If it has four bonds (and no lone pair), it has a formal. How to calculate formal charge. 3 for boron, 4 for carbon, 5 for nitrogen, and so on). To calculate the formal charge of an atom, we start by: In the left resonance structure, all the atoms have zero formal charge, while on the right structure, the nitrogen has a +1 formal charge, and the. So now let’s calculate the formal charge on each individual atom present in no2. Evaluating the number of valence electrons (ve) the neutral atom has (e.g. If a nitrogen has three bonds and a lone pair, it has a formal charge of zero. Here nitrogen is the free atom and the number of valence.
From www.chegg.com
Solved What is the formal charge of nitrogen in the Nitrogen In No2 Formal Charge If a nitrogen has three bonds and a lone pair, it has a formal charge of zero. N = o.around each atom from your left to right there are 9, 7, and 8 electrons respectively leading to formal charges of −1, 0, and 0. Formal charge on nitrogen atom: Evaluating the number of valence electrons (ve) the neutral atom has. Nitrogen In No2 Formal Charge.
From www.numerade.com
What is the formal charge of the nitrogen atom in the molecule shown below? AH Nitrogen In No2 Formal Charge N = o.around each atom from your left to right there are 9, 7, and 8 electrons respectively leading to formal charges of −1, 0, and 0. If it has four bonds (and no lone pair), it has a formal. To calculate the formal charge of an atom, we start by: How to calculate formal charge. If a nitrogen has. Nitrogen In No2 Formal Charge.
From chemistry291.blogspot.com
N2 Lewis Structure ,Valence Electrons,Formal Charge,Polar or Non polar Nitrogen In No2 Formal Charge So now let’s calculate the formal charge on each individual atom present in no2. N = o.around each atom from your left to right there are 9, 7, and 8 electrons respectively leading to formal charges of −1, 0, and 0. 3 for boron, 4 for carbon, 5 for nitrogen, and so on). In the left resonance structure, all the. Nitrogen In No2 Formal Charge.
From brainly.com
Write the Lewis dot structure for the No2 molecule and calculate the formal charge on each Nitrogen In No2 Formal Charge 3 for boron, 4 for carbon, 5 for nitrogen, and so on). If a nitrogen has three bonds and a lone pair, it has a formal charge of zero. Formal charge on nitrogen atom: In the left resonance structure, all the atoms have zero formal charge, while on the right structure, the nitrogen has a +1 formal charge, and the.. Nitrogen In No2 Formal Charge.
From www.numerade.com
SOLVED What is the formal charge on nitrogen in the structure below? 1 0 ONo 1 +1 Nitrogen In No2 Formal Charge Here nitrogen is the free atom and the number of valence. In the left resonance structure, all the atoms have zero formal charge, while on the right structure, the nitrogen has a +1 formal charge, and the. 3 for boron, 4 for carbon, 5 for nitrogen, and so on). N = o.around each atom from your left to right there. Nitrogen In No2 Formal Charge.
From stock.adobe.com
Chemical formulas of nitrogen oxide nitric oxide NO, nitrogen dioxide NO2, nitrous oxide N2O Nitrogen In No2 Formal Charge How to calculate formal charge. Here nitrogen is the free atom and the number of valence. So now let’s calculate the formal charge on each individual atom present in no2. If it has four bonds (and no lone pair), it has a formal. N = o.around each atom from your left to right there are 9, 7, and 8 electrons. Nitrogen In No2 Formal Charge.
From www.youtube.com
Resonance Structures for NO2 (Nitrogen dioxide) YouTube Nitrogen In No2 Formal Charge So now let’s calculate the formal charge on each individual atom present in no2. Here nitrogen is the free atom and the number of valence. If it has four bonds (and no lone pair), it has a formal. Continuing with the nitrogen, we observe that in (a) the nitrogen atom shares three bonding pairs and has one lone pair and. Nitrogen In No2 Formal Charge.
From socratic.org
How to count formal charge in NO2? Socratic Nitrogen In No2 Formal Charge 3 for boron, 4 for carbon, 5 for nitrogen, and so on). How to calculate formal charge. If a nitrogen has three bonds and a lone pair, it has a formal charge of zero. In the left resonance structure, all the atoms have zero formal charge, while on the right structure, the nitrogen has a +1 formal charge, and the.. Nitrogen In No2 Formal Charge.
From www.coursehero.com
[Solved] What is the formal charge on the central nitrogen atom in the most... Course Hero Nitrogen In No2 Formal Charge Evaluating the number of valence electrons (ve) the neutral atom has (e.g. Here nitrogen is the free atom and the number of valence. 3 for boron, 4 for carbon, 5 for nitrogen, and so on). N = o.around each atom from your left to right there are 9, 7, and 8 electrons respectively leading to formal charges of −1, 0,. Nitrogen In No2 Formal Charge.
From www.youtube.com
Formal Charge Key Patterns for Carbon, Nitrogen, Oxygen YouTube Nitrogen In No2 Formal Charge If a nitrogen has three bonds and a lone pair, it has a formal charge of zero. 3 for boron, 4 for carbon, 5 for nitrogen, and so on). Continuing with the nitrogen, we observe that in (a) the nitrogen atom shares three bonding pairs and has one lone pair and has a total of 5 valence. In the left. Nitrogen In No2 Formal Charge.
From www.youtube.com
NO2 Lewis Structure How to Draw the Lewis Structure for NO2 YouTube Nitrogen In No2 Formal Charge Formal charge on nitrogen atom: 3 for boron, 4 for carbon, 5 for nitrogen, and so on). Evaluating the number of valence electrons (ve) the neutral atom has (e.g. N = o.around each atom from your left to right there are 9, 7, and 8 electrons respectively leading to formal charges of −1, 0, and 0. How to calculate formal. Nitrogen In No2 Formal Charge.
From animalia-life.club
Resonance Structures No2 Nitrogen In No2 Formal Charge If a nitrogen has three bonds and a lone pair, it has a formal charge of zero. So now let’s calculate the formal charge on each individual atom present in no2. N = o.around each atom from your left to right there are 9, 7, and 8 electrons respectively leading to formal charges of −1, 0, and 0. Formal charge. Nitrogen In No2 Formal Charge.
From www.slideserve.com
PPT Entry Task Oct 22 nd Monday PowerPoint Presentation, free download ID6280929 Nitrogen In No2 Formal Charge Formal charge on nitrogen atom: 3 for boron, 4 for carbon, 5 for nitrogen, and so on). To calculate the formal charge of an atom, we start by: N = o.around each atom from your left to right there are 9, 7, and 8 electrons respectively leading to formal charges of −1, 0, and 0. If a nitrogen has three. Nitrogen In No2 Formal Charge.
From www.numerade.com
SOLVED Calculate the formal charge on the indicated atom in each of the following molecules or Nitrogen In No2 Formal Charge Evaluating the number of valence electrons (ve) the neutral atom has (e.g. 3 for boron, 4 for carbon, 5 for nitrogen, and so on). If it has four bonds (and no lone pair), it has a formal. Continuing with the nitrogen, we observe that in (a) the nitrogen atom shares three bonding pairs and has one lone pair and has. Nitrogen In No2 Formal Charge.
From www.chegg.com
Solved What is the formal charge on nitrogen in the Nitrogen In No2 Formal Charge If it has four bonds (and no lone pair), it has a formal. Formal charge on nitrogen atom: 3 for boron, 4 for carbon, 5 for nitrogen, and so on). So now let’s calculate the formal charge on each individual atom present in no2. Evaluating the number of valence electrons (ve) the neutral atom has (e.g. Here nitrogen is the. Nitrogen In No2 Formal Charge.
From truyenhinhcapsongthu.net
How To Calculate Formal Charge Master Organic Chemistry Nitrogen In No2 Formal Charge If a nitrogen has three bonds and a lone pair, it has a formal charge of zero. In the left resonance structure, all the atoms have zero formal charge, while on the right structure, the nitrogen has a +1 formal charge, and the. If it has four bonds (and no lone pair), it has a formal. Evaluating the number of. Nitrogen In No2 Formal Charge.
From www.chegg.com
Solved What are the formal charges of the two nitrogen atoms Nitrogen In No2 Formal Charge Formal charge on nitrogen atom: Continuing with the nitrogen, we observe that in (a) the nitrogen atom shares three bonding pairs and has one lone pair and has a total of 5 valence. Evaluating the number of valence electrons (ve) the neutral atom has (e.g. In the left resonance structure, all the atoms have zero formal charge, while on the. Nitrogen In No2 Formal Charge.
From www.numerade.com
SOLVED Calculate the formal charge on nitrogen in CH3NO2 (Hint Lewis structure) 0 1 Nitrogen In No2 Formal Charge How to calculate formal charge. To calculate the formal charge of an atom, we start by: Formal charge on nitrogen atom: In the left resonance structure, all the atoms have zero formal charge, while on the right structure, the nitrogen has a +1 formal charge, and the. So now let’s calculate the formal charge on each individual atom present in. Nitrogen In No2 Formal Charge.
From www.chegg.com
Solved Determine the formal charge on the nitrogen atom in Nitrogen In No2 Formal Charge 3 for boron, 4 for carbon, 5 for nitrogen, and so on). Here nitrogen is the free atom and the number of valence. To calculate the formal charge of an atom, we start by: In the left resonance structure, all the atoms have zero formal charge, while on the right structure, the nitrogen has a +1 formal charge, and the.. Nitrogen In No2 Formal Charge.
From www.numerade.com
SOLVED The formal charge on the nitrogen atom in the resonance structure of nitrate (NO3) is Nitrogen In No2 Formal Charge 3 for boron, 4 for carbon, 5 for nitrogen, and so on). Here nitrogen is the free atom and the number of valence. How to calculate formal charge. N = o.around each atom from your left to right there are 9, 7, and 8 electrons respectively leading to formal charges of −1, 0, and 0. So now let’s calculate the. Nitrogen In No2 Formal Charge.
From www.youtube.com
Formal charge class 11,12 chemistry formal charge in ozon , carbonate ion , nitrogen dioxide Nitrogen In No2 Formal Charge Evaluating the number of valence electrons (ve) the neutral atom has (e.g. To calculate the formal charge of an atom, we start by: Here nitrogen is the free atom and the number of valence. If it has four bonds (and no lone pair), it has a formal. In the left resonance structure, all the atoms have zero formal charge, while. Nitrogen In No2 Formal Charge.
From www.chegg.com
Solved The formal charge on the nitrogen atom in nitrite ion Nitrogen In No2 Formal Charge How to calculate formal charge. To calculate the formal charge of an atom, we start by: If a nitrogen has three bonds and a lone pair, it has a formal charge of zero. So now let’s calculate the formal charge on each individual atom present in no2. Here nitrogen is the free atom and the number of valence. Continuing with. Nitrogen In No2 Formal Charge.
From www.youtube.com
Formal charge of NO2 Formal charge of Nitrite ion YouTube Nitrogen In No2 Formal Charge If a nitrogen has three bonds and a lone pair, it has a formal charge of zero. How to calculate formal charge. In the left resonance structure, all the atoms have zero formal charge, while on the right structure, the nitrogen has a +1 formal charge, and the. Evaluating the number of valence electrons (ve) the neutral atom has (e.g.. Nitrogen In No2 Formal Charge.
From www.numerade.com
SOLVED The lewis structure of a nitrite Ion (NO2") is given below 8 N=8 Calculate the formal Nitrogen In No2 Formal Charge To calculate the formal charge of an atom, we start by: How to calculate formal charge. In the left resonance structure, all the atoms have zero formal charge, while on the right structure, the nitrogen has a +1 formal charge, and the. 3 for boron, 4 for carbon, 5 for nitrogen, and so on). If a nitrogen has three bonds. Nitrogen In No2 Formal Charge.
From truyenhinhcapsongthu.net
How To Calculate Formal Charge Master Organic Chemistry Nitrogen In No2 Formal Charge To calculate the formal charge of an atom, we start by: Continuing with the nitrogen, we observe that in (a) the nitrogen atom shares three bonding pairs and has one lone pair and has a total of 5 valence. If a nitrogen has three bonds and a lone pair, it has a formal charge of zero. Formal charge on nitrogen. Nitrogen In No2 Formal Charge.
From www.reddit.com
confused as to placement of positive charge in NO2+ lewis structure. the + is assigned to the Nitrogen In No2 Formal Charge Formal charge on nitrogen atom: In the left resonance structure, all the atoms have zero formal charge, while on the right structure, the nitrogen has a +1 formal charge, and the. Evaluating the number of valence electrons (ve) the neutral atom has (e.g. How to calculate formal charge. Continuing with the nitrogen, we observe that in (a) the nitrogen atom. Nitrogen In No2 Formal Charge.
From solvedlib.com
What is the formal charge of 'nitrogen in this s… SolvedLib Nitrogen In No2 Formal Charge 3 for boron, 4 for carbon, 5 for nitrogen, and so on). Evaluating the number of valence electrons (ve) the neutral atom has (e.g. If a nitrogen has three bonds and a lone pair, it has a formal charge of zero. N = o.around each atom from your left to right there are 9, 7, and 8 electrons respectively leading. Nitrogen In No2 Formal Charge.
From www.chegg.com
Solved The formal charge on the nitrogen atom in the Nitrogen In No2 Formal Charge N = o.around each atom from your left to right there are 9, 7, and 8 electrons respectively leading to formal charges of −1, 0, and 0. If it has four bonds (and no lone pair), it has a formal. Continuing with the nitrogen, we observe that in (a) the nitrogen atom shares three bonding pairs and has one lone. Nitrogen In No2 Formal Charge.
From clarkfvspears.blogspot.com
How to Calculate Formal Charge ClarkfvSpears Nitrogen In No2 Formal Charge Evaluating the number of valence electrons (ve) the neutral atom has (e.g. If it has four bonds (and no lone pair), it has a formal. N = o.around each atom from your left to right there are 9, 7, and 8 electrons respectively leading to formal charges of −1, 0, and 0. Formal charge on nitrogen atom: To calculate the. Nitrogen In No2 Formal Charge.
From www.youtube.com
How to Calculate the Formal Charges for NF3 (Nitrogen trifluoride) YouTube Nitrogen In No2 Formal Charge If a nitrogen has three bonds and a lone pair, it has a formal charge of zero. If it has four bonds (and no lone pair), it has a formal. In the left resonance structure, all the atoms have zero formal charge, while on the right structure, the nitrogen has a +1 formal charge, and the. Formal charge on nitrogen. Nitrogen In No2 Formal Charge.
From www.answersarena.com
[Solved] Determine the formal charge of nitrogen in the s Nitrogen In No2 Formal Charge In the left resonance structure, all the atoms have zero formal charge, while on the right structure, the nitrogen has a +1 formal charge, and the. N = o.around each atom from your left to right there are 9, 7, and 8 electrons respectively leading to formal charges of −1, 0, and 0. 3 for boron, 4 for carbon, 5. Nitrogen In No2 Formal Charge.
From www.dreamstime.com
Nitrogen Dioxide, NO2 Molecule. Structural Chemical Formula and Molecule Model Stock Vector Nitrogen In No2 Formal Charge Formal charge on nitrogen atom: If it has four bonds (and no lone pair), it has a formal. In the left resonance structure, all the atoms have zero formal charge, while on the right structure, the nitrogen has a +1 formal charge, and the. If a nitrogen has three bonds and a lone pair, it has a formal charge of. Nitrogen In No2 Formal Charge.
From www.vrogue.co
What Is The Valency Of Nitrogen In No2 Chemistry Atom vrogue.co Nitrogen In No2 Formal Charge If it has four bonds (and no lone pair), it has a formal. How to calculate formal charge. To calculate the formal charge of an atom, we start by: 3 for boron, 4 for carbon, 5 for nitrogen, and so on). In the left resonance structure, all the atoms have zero formal charge, while on the right structure, the nitrogen. Nitrogen In No2 Formal Charge.
From www.slideserve.com
PPT Both bonding electrons remain with one product fragment in this kind of reaction Nitrogen In No2 Formal Charge Here nitrogen is the free atom and the number of valence. In the left resonance structure, all the atoms have zero formal charge, while on the right structure, the nitrogen has a +1 formal charge, and the. Evaluating the number of valence electrons (ve) the neutral atom has (e.g. Formal charge on nitrogen atom: To calculate the formal charge of. Nitrogen In No2 Formal Charge.
From www.youtube.com
How to Calculate the Formal Charges for N2O (Nitrous oxide or Dinitrogen oxide) YouTube Nitrogen In No2 Formal Charge If it has four bonds (and no lone pair), it has a formal. To calculate the formal charge of an atom, we start by: Evaluating the number of valence electrons (ve) the neutral atom has (e.g. Continuing with the nitrogen, we observe that in (a) the nitrogen atom shares three bonding pairs and has one lone pair and has a. Nitrogen In No2 Formal Charge.