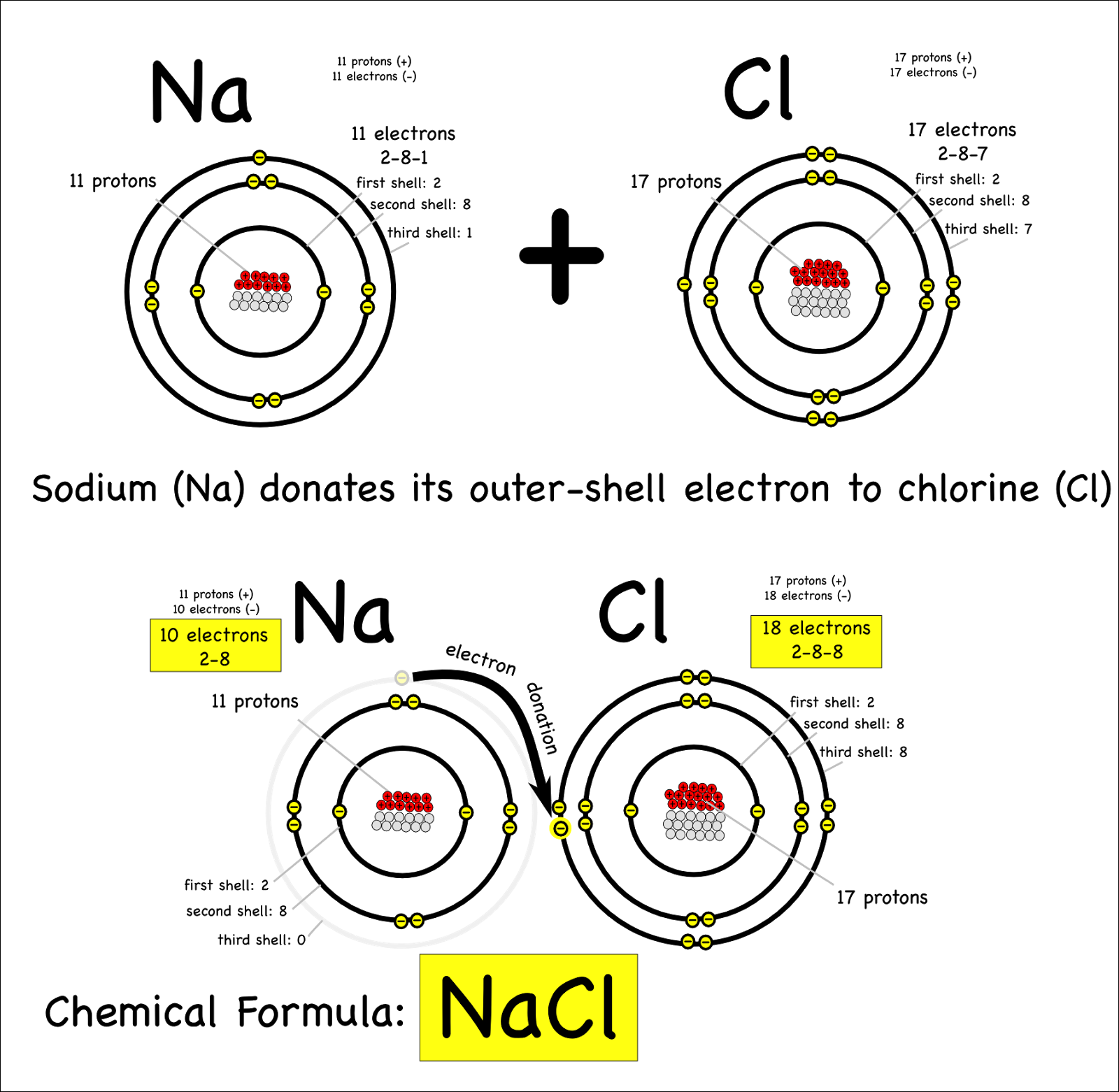
Chlorine Ionic Or Covalent Bond . The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). The best guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in the. Many bonds can be covalent in one situation and ionic in another. Ionic compounds generally tend to have higher melting and boiling points, covalent compounds have lower melting & boiling points Ionic bonds tend to be strong, forming ionic crystals that are hard and brittle. Ionic bonds generally tend to transfer electrons, covalent bonds share them more easily; For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and chlorine are covalently bound, but if hcl is. A good example of an ionic bond is the bond between the sodium atom and chlorine atom in sodium. Let’s look at some differences between ionic and covalent bonds and compounds.
from fourthgradegc.blogspot.com
Ionic compounds generally tend to have higher melting and boiling points, covalent compounds have lower melting & boiling points The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). A good example of an ionic bond is the bond between the sodium atom and chlorine atom in sodium. Let’s look at some differences between ionic and covalent bonds and compounds. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and chlorine are covalently bound, but if hcl is. Ionic bonds tend to be strong, forming ionic crystals that are hard and brittle. Ionic bonds generally tend to transfer electrons, covalent bonds share them more easily; The best guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in the. Many bonds can be covalent in one situation and ionic in another.
Fourth Grade GC August 2013
Chlorine Ionic Or Covalent Bond The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). Ionic compounds generally tend to have higher melting and boiling points, covalent compounds have lower melting & boiling points For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and chlorine are covalently bound, but if hcl is. The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). Ionic bonds tend to be strong, forming ionic crystals that are hard and brittle. Let’s look at some differences between ionic and covalent bonds and compounds. Ionic bonds generally tend to transfer electrons, covalent bonds share them more easily; A good example of an ionic bond is the bond between the sodium atom and chlorine atom in sodium. Many bonds can be covalent in one situation and ionic in another. The best guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in the.
From www.chemistrystudent.com
Covalent Bonding (ALevel) ChemistryStudent Chlorine Ionic Or Covalent Bond Ionic bonds generally tend to transfer electrons, covalent bonds share them more easily; A good example of an ionic bond is the bond between the sodium atom and chlorine atom in sodium. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and chlorine are covalently bound, but if hcl is. Ionic compounds generally tend to have higher. Chlorine Ionic Or Covalent Bond.
From sciencenotes.org
Covalent Compounds Examples and Properties Chlorine Ionic Or Covalent Bond Ionic bonds tend to be strong, forming ionic crystals that are hard and brittle. The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). The best guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions. Chlorine Ionic Or Covalent Bond.
From www.expii.com
Ionic Bonding (Biology) — Definition & Role Expii Chlorine Ionic Or Covalent Bond Ionic compounds generally tend to have higher melting and boiling points, covalent compounds have lower melting & boiling points The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). Ionic bonds generally tend to transfer electrons, covalent bonds share them more easily; Ionic bonds tend to be strong,. Chlorine Ionic Or Covalent Bond.
From www.differencebetween.com
Difference Between Ionic and Covalent Bonds Compare the Difference Chlorine Ionic Or Covalent Bond A good example of an ionic bond is the bond between the sodium atom and chlorine atom in sodium. The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). Ionic bonds generally tend to transfer electrons, covalent bonds share them more easily; Let’s look at some differences between. Chlorine Ionic Or Covalent Bond.
From surfguppy.com
What is Ionic Bond Surfguppy Chemistry made easy visual learning Chlorine Ionic Or Covalent Bond Ionic bonds tend to be strong, forming ionic crystals that are hard and brittle. Ionic bonds generally tend to transfer electrons, covalent bonds share them more easily; Ionic compounds generally tend to have higher melting and boiling points, covalent compounds have lower melting & boiling points For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and chlorine. Chlorine Ionic Or Covalent Bond.
From www.vedantu.com
Chemistry Chapter 4 Chemical Bonding Class 11 Notes Chlorine Ionic Or Covalent Bond The best guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in the. A good example of an ionic bond is the bond between the sodium atom and chlorine atom in sodium. The degree to which electrons are shared between atoms varies from completely equal (pure covalent. Chlorine Ionic Or Covalent Bond.
From fourthgradegc.blogspot.com
Fourth Grade GC August 2013 Chlorine Ionic Or Covalent Bond Ionic bonds generally tend to transfer electrons, covalent bonds share them more easily; Ionic bonds tend to be strong, forming ionic crystals that are hard and brittle. Many bonds can be covalent in one situation and ionic in another. Let’s look at some differences between ionic and covalent bonds and compounds. A good example of an ionic bond is the. Chlorine Ionic Or Covalent Bond.
From brainly.com
The diatomic molecule of chlorine, cl2, is held together by a(n Chlorine Ionic Or Covalent Bond Ionic bonds tend to be strong, forming ionic crystals that are hard and brittle. A good example of an ionic bond is the bond between the sodium atom and chlorine atom in sodium. Ionic compounds generally tend to have higher melting and boiling points, covalent compounds have lower melting & boiling points Many bonds can be covalent in one situation. Chlorine Ionic Or Covalent Bond.
From www.shalom-education.com
Covalent Bonding GCSE Chemistry Revision Chlorine Ionic Or Covalent Bond Let’s look at some differences between ionic and covalent bonds and compounds. Ionic compounds generally tend to have higher melting and boiling points, covalent compounds have lower melting & boiling points Many bonds can be covalent in one situation and ionic in another. The best guide to the covalent or ionic character of a bond is to consider the types. Chlorine Ionic Or Covalent Bond.
From philschatz.com
Chemical Bonds · Anatomy and Physiology Chlorine Ionic Or Covalent Bond The best guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in the. Many bonds can be covalent in one situation and ionic in another. Ionic compounds generally tend to have higher melting and boiling points, covalent compounds have lower melting & boiling points A good example. Chlorine Ionic Or Covalent Bond.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Chlorine Ionic Or Covalent Bond For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and chlorine are covalently bound, but if hcl is. Let’s look at some differences between ionic and covalent bonds and compounds. Many bonds can be covalent in one situation and ionic in another. A good example of an ionic bond is the bond between the sodium atom and. Chlorine Ionic Or Covalent Bond.
From en.wikipedia.org
Ionic bonding Wikipedia Chlorine Ionic Or Covalent Bond Let’s look at some differences between ionic and covalent bonds and compounds. Ionic bonds generally tend to transfer electrons, covalent bonds share them more easily; The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). Many bonds can be covalent in one situation and ionic in another. Ionic. Chlorine Ionic Or Covalent Bond.
From slidesharenow.blogspot.com
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare Chlorine Ionic Or Covalent Bond For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and chlorine are covalently bound, but if hcl is. Ionic bonds generally tend to transfer electrons, covalent bonds share them more easily; Ionic bonds tend to be strong, forming ionic crystals that are hard and brittle. The degree to which electrons are shared between atoms varies from completely. Chlorine Ionic Or Covalent Bond.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Chlorine Ionic Or Covalent Bond Let’s look at some differences between ionic and covalent bonds and compounds. Ionic bonds generally tend to transfer electrons, covalent bonds share them more easily; Ionic compounds generally tend to have higher melting and boiling points, covalent compounds have lower melting & boiling points Ionic bonds tend to be strong, forming ionic crystals that are hard and brittle. For instance,. Chlorine Ionic Or Covalent Bond.
From www.youtube.com
Is Cl2 (Chlorine gas) ionic or covalent? YouTube Chlorine Ionic Or Covalent Bond The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). A good example of an ionic bond is the bond between the sodium atom and chlorine atom in sodium. Ionic bonds tend to be strong, forming ionic crystals that are hard and brittle. Many bonds can be covalent. Chlorine Ionic Or Covalent Bond.
From slideplayer.com
Describe the function of the following Helper T cells ppt download Chlorine Ionic Or Covalent Bond The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). A good example of an ionic bond is the bond between the sodium atom and chlorine atom in sodium. Let’s look at some differences between ionic and covalent bonds and compounds. The best guide to the covalent or. Chlorine Ionic Or Covalent Bond.
From chemistry98.blogspot.com
Chem Easy Formation of covalent bond in chlorine molecule Chlorine Ionic Or Covalent Bond Ionic bonds tend to be strong, forming ionic crystals that are hard and brittle. Let’s look at some differences between ionic and covalent bonds and compounds. The best guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in the. For instance, hydrogen chloride, hcl, is a gas. Chlorine Ionic Or Covalent Bond.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Chlorine Ionic Or Covalent Bond The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). A good example of an ionic bond is the bond between the sodium atom and chlorine atom in sodium. Ionic bonds tend to be strong, forming ionic crystals that are hard and brittle. Many bonds can be covalent. Chlorine Ionic Or Covalent Bond.
From fineartamerica.com
Bond Formation In Chlorine Molecule Photograph by Chlorine Ionic Or Covalent Bond The best guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in the. Many bonds can be covalent in one situation and ionic in another. The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding).. Chlorine Ionic Or Covalent Bond.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Chlorine Ionic Or Covalent Bond Let’s look at some differences between ionic and covalent bonds and compounds. Ionic bonds tend to be strong, forming ionic crystals that are hard and brittle. Ionic bonds generally tend to transfer electrons, covalent bonds share them more easily; For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and chlorine are covalently bound, but if hcl is.. Chlorine Ionic Or Covalent Bond.
From www.alamy.com
Diagram to show ionic bonding in sodium chloride Stock Vector Image Chlorine Ionic Or Covalent Bond For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and chlorine are covalently bound, but if hcl is. Ionic compounds generally tend to have higher melting and boiling points, covalent compounds have lower melting & boiling points A good example of an ionic bond is the bond between the sodium atom and chlorine atom in sodium. Ionic. Chlorine Ionic Or Covalent Bond.
From chemistrylearnwithsangam.blogspot.com
chemistry knowledge Comparison between Covalent and Ionic Bond Chlorine Ionic Or Covalent Bond A good example of an ionic bond is the bond between the sodium atom and chlorine atom in sodium. Ionic bonds generally tend to transfer electrons, covalent bonds share them more easily; Let’s look at some differences between ionic and covalent bonds and compounds. Ionic bonds tend to be strong, forming ionic crystals that are hard and brittle. The best. Chlorine Ionic Or Covalent Bond.
From biologydictionary.net
Covalent Bond Biology Dictionary Chlorine Ionic Or Covalent Bond Ionic bonds tend to be strong, forming ionic crystals that are hard and brittle. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and chlorine are covalently bound, but if hcl is. Ionic compounds generally tend to have higher melting and boiling points, covalent compounds have lower melting & boiling points Let’s look at some differences between. Chlorine Ionic Or Covalent Bond.
From biologydictionary.net
Ionic Bond Examples Biology Dictionary Chlorine Ionic Or Covalent Bond The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). Let’s look at some differences between ionic and covalent bonds and compounds. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and chlorine are covalently bound, but if hcl is. Many bonds can be covalent. Chlorine Ionic Or Covalent Bond.
From thisonevsthatone.com
Ionic vs Covalent Which is which and how to tell them apart Chlorine Ionic Or Covalent Bond Let’s look at some differences between ionic and covalent bonds and compounds. Ionic bonds generally tend to transfer electrons, covalent bonds share them more easily; The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and. Chlorine Ionic Or Covalent Bond.
From www.alamy.com
Covalent bond of hydrogen chlorine (HCl). Physics resources for Chlorine Ionic Or Covalent Bond Let’s look at some differences between ionic and covalent bonds and compounds. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and chlorine are covalently bound, but if hcl is. Many bonds can be covalent in one situation and ionic in another. The degree to which electrons are shared between atoms varies from completely equal (pure covalent. Chlorine Ionic Or Covalent Bond.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and Chlorine Ionic Or Covalent Bond A good example of an ionic bond is the bond between the sodium atom and chlorine atom in sodium. Many bonds can be covalent in one situation and ionic in another. Ionic compounds generally tend to have higher melting and boiling points, covalent compounds have lower melting & boiling points The degree to which electrons are shared between atoms varies. Chlorine Ionic Or Covalent Bond.
From brainly.com
This image shows the ionic bond between sodium and chloride in NaCl Chlorine Ionic Or Covalent Bond A good example of an ionic bond is the bond between the sodium atom and chlorine atom in sodium. Let’s look at some differences between ionic and covalent bonds and compounds. Many bonds can be covalent in one situation and ionic in another. Ionic bonds tend to be strong, forming ionic crystals that are hard and brittle. The degree to. Chlorine Ionic Or Covalent Bond.
From www.teachoo.com
[Class 10] Differentiate between Ionic bond & Covalent bond Teachoo Chlorine Ionic Or Covalent Bond The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). Ionic bonds tend to be strong, forming ionic crystals that are hard and brittle. Let’s look at some differences between ionic and covalent bonds and compounds. A good example of an ionic bond is the bond between the. Chlorine Ionic Or Covalent Bond.
From oerpub.github.io
The top panel of this figure shows the orbit model of a sodium atom and Chlorine Ionic Or Covalent Bond Many bonds can be covalent in one situation and ionic in another. The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). Ionic compounds generally tend to have higher melting and boiling points, covalent compounds have lower melting & boiling points Ionic bonds generally tend to transfer electrons,. Chlorine Ionic Or Covalent Bond.
From www.pinterest.ca
Covalent bond vector illustration. Process explanation labeled diagram Chlorine Ionic Or Covalent Bond For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and chlorine are covalently bound, but if hcl is. The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). The best guide to the covalent or ionic character of a bond is to consider the types. Chlorine Ionic Or Covalent Bond.
From www.slideserve.com
PPT A Case Study on Covalent Bonding PowerPoint Presentation, free Chlorine Ionic Or Covalent Bond Many bonds can be covalent in one situation and ionic in another. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and chlorine are covalently bound, but if hcl is. Ionic compounds generally tend to have higher melting and boiling points, covalent compounds have lower melting & boiling points Let’s look at some differences between ionic and. Chlorine Ionic Or Covalent Bond.
From slidetodoc.com
Presentation ON Ionic Covalent and Metallic bonding B Chlorine Ionic Or Covalent Bond Let’s look at some differences between ionic and covalent bonds and compounds. The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and chlorine are covalently bound, but if hcl is. Ionic compounds generally tend to. Chlorine Ionic Or Covalent Bond.
From thesciencecore.blogspot.com
covalent bond definition, properties and examples The Science Core Chlorine Ionic Or Covalent Bond Ionic compounds generally tend to have higher melting and boiling points, covalent compounds have lower melting & boiling points Ionic bonds generally tend to transfer electrons, covalent bonds share them more easily; A good example of an ionic bond is the bond between the sodium atom and chlorine atom in sodium. The degree to which electrons are shared between atoms. Chlorine Ionic Or Covalent Bond.
From mammothmemory.net
Chemical bonding is about atoms achieving full outer shells Chlorine Ionic Or Covalent Bond Ionic compounds generally tend to have higher melting and boiling points, covalent compounds have lower melting & boiling points A good example of an ionic bond is the bond between the sodium atom and chlorine atom in sodium. Ionic bonds tend to be strong, forming ionic crystals that are hard and brittle. Many bonds can be covalent in one situation. Chlorine Ionic Or Covalent Bond.