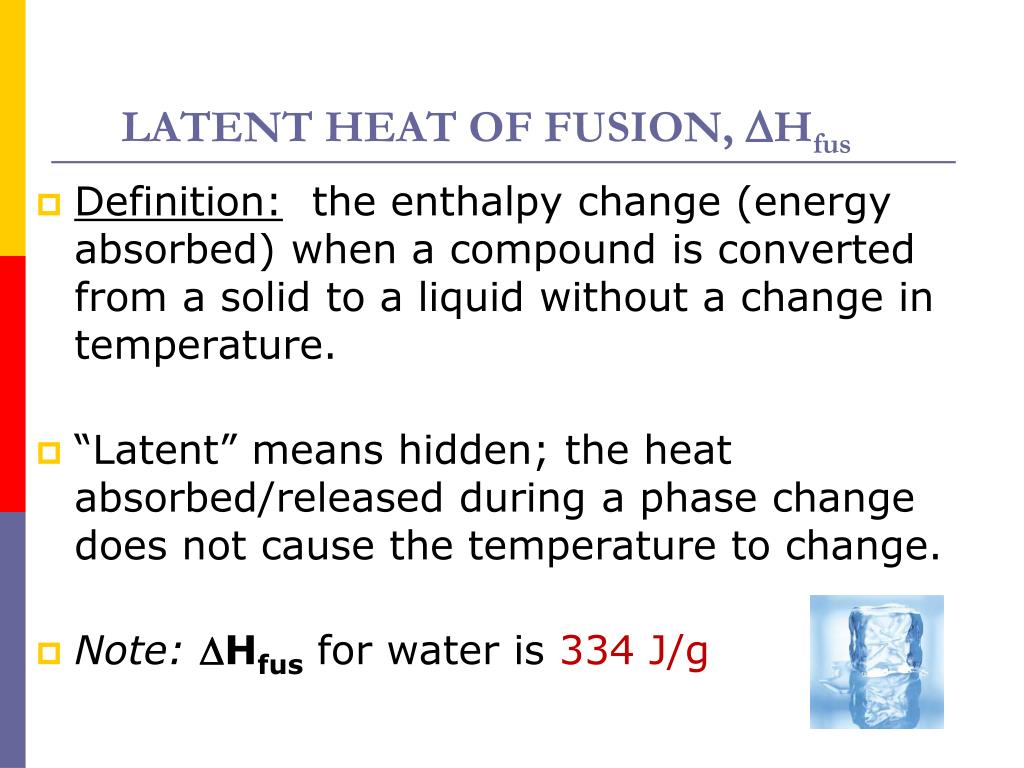
Heat Fusion Definition . Some liquids can be supercooled—i.e., cooled below. This process is better known as melting, or heat of fusion, and results in the molecules within the substance becoming less. The molar heat of solidification (δhsolid). The heat of fusion definition communicates a form of energy that is either released or absorbed when a certain amount of matter is changing phase. The heat of fusion is alternatively known. The heat of fusion is the amount of energy required to change a substance from a solid to a liquid at its melting point without. The concept of heat of fusion, often denoted by the symbol δh fus, is a critical parameter in thermodynamics and material. What is heat of fusion? The heat of fusion (see thermal fusion), the heat that must be applied to melt a solid, must be removed from the liquid to freeze it. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant. The molar heat of fusion (δhfus) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to a liquid.
from www.slideserve.com
This process is better known as melting, or heat of fusion, and results in the molecules within the substance becoming less. What is heat of fusion? The heat of fusion is alternatively known. The heat of fusion (see thermal fusion), the heat that must be applied to melt a solid, must be removed from the liquid to freeze it. The molar heat of solidification (δhsolid). The heat of fusion is the amount of energy required to change a substance from a solid to a liquid at its melting point without. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant. The heat of fusion definition communicates a form of energy that is either released or absorbed when a certain amount of matter is changing phase. The molar heat of fusion (δhfus) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to a liquid. The concept of heat of fusion, often denoted by the symbol δh fus, is a critical parameter in thermodynamics and material.
PPT Thermochemistry PowerPoint Presentation, free download ID2841621
Heat Fusion Definition The molar heat of solidification (δhsolid). The heat of fusion is alternatively known. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant. The heat of fusion definition communicates a form of energy that is either released or absorbed when a certain amount of matter is changing phase. The molar heat of fusion (δhfus) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to a liquid. The molar heat of solidification (δhsolid). The concept of heat of fusion, often denoted by the symbol δh fus, is a critical parameter in thermodynamics and material. This process is better known as melting, or heat of fusion, and results in the molecules within the substance becoming less. The heat of fusion (see thermal fusion), the heat that must be applied to melt a solid, must be removed from the liquid to freeze it. What is heat of fusion? Some liquids can be supercooled—i.e., cooled below. The heat of fusion is the amount of energy required to change a substance from a solid to a liquid at its melting point without.
From www.youtube.com
Specific heat, heat of fusion and vaporization example Chemistry Heat Fusion Definition The heat of fusion (see thermal fusion), the heat that must be applied to melt a solid, must be removed from the liquid to freeze it. Some liquids can be supercooled—i.e., cooled below. This process is better known as melting, or heat of fusion, and results in the molecules within the substance becoming less. The molar heat of fusion (δhfus). Heat Fusion Definition.
From www.youtube.com
Latent heat of fusion Class 9 Physics Chapter 8 YouTube Heat Fusion Definition This process is better known as melting, or heat of fusion, and results in the molecules within the substance becoming less. What is heat of fusion? The molar heat of solidification (δhsolid). The heat of fusion definition communicates a form of energy that is either released or absorbed when a certain amount of matter is changing phase. The molar heat. Heat Fusion Definition.
From www.ck12.org
Heat of fusion Example 1 ( Video ) Chemistry CK12 Foundation Heat Fusion Definition The heat of fusion definition communicates a form of energy that is either released or absorbed when a certain amount of matter is changing phase. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant. This process is better known as. Heat Fusion Definition.
From www.teachoo.com
Latent Heat of Vaporization and Fusion Definition Teachoo Heat Fusion Definition This process is better known as melting, or heat of fusion, and results in the molecules within the substance becoming less. The molar heat of fusion (δhfus) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to a liquid. The concept of heat of fusion, often denoted by the. Heat Fusion Definition.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2841621 Heat Fusion Definition The concept of heat of fusion, often denoted by the symbol δh fus, is a critical parameter in thermodynamics and material. What is heat of fusion? Some liquids can be supercooled—i.e., cooled below. The heat of fusion is alternatively known. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to. Heat Fusion Definition.
From www.energy.gov
Infographic Fast Facts on Fusion Department of Energy Heat Fusion Definition Some liquids can be supercooled—i.e., cooled below. The molar heat of solidification (δhsolid). The heat of fusion is alternatively known. The molar heat of fusion (δhfus) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to a liquid. The heat of fusion (see thermal fusion), the heat that must. Heat Fusion Definition.
From www.slideserve.com
PPT Energy and Chemical Change PowerPoint Presentation ID1278045 Heat Fusion Definition Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant. This process is better known as melting, or heat of fusion, and results in the molecules within the substance becoming less. What is heat of fusion? The concept of heat of. Heat Fusion Definition.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Heat Fusion Definition Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant. This process is better known as melting, or heat of fusion, and results in the molecules within the substance becoming less. The heat of fusion definition communicates a form of energy. Heat Fusion Definition.
From apollo.lsc.vsc.edu
Latent Heat of evaporation, fusion, and freezing Heat Fusion Definition The heat of fusion (see thermal fusion), the heat that must be applied to melt a solid, must be removed from the liquid to freeze it. The molar heat of fusion (δhfus) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to a liquid. Some liquids can be supercooled—i.e.,. Heat Fusion Definition.
From www.geeksforgeeks.org
Latent Heat of Fusion Definition, Formula, Examples & FAQs Heat Fusion Definition Some liquids can be supercooled—i.e., cooled below. The heat of fusion definition communicates a form of energy that is either released or absorbed when a certain amount of matter is changing phase. The heat of fusion is the amount of energy required to change a substance from a solid to a liquid at its melting point without. Heat of fusion,. Heat Fusion Definition.
From www.ck12.org
Heat of fusion Example 2 ( Video ) Chemistry CK12 Foundation Heat Fusion Definition The heat of fusion (see thermal fusion), the heat that must be applied to melt a solid, must be removed from the liquid to freeze it. The heat of fusion is alternatively known. The molar heat of fusion (δhfus) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to. Heat Fusion Definition.
From www.slideserve.com
PPT Heat of Reactions PowerPoint Presentation, free download ID5747139 Heat Fusion Definition The molar heat of fusion (δhfus) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to a liquid. The molar heat of solidification (δhsolid). The heat of fusion is alternatively known. What is heat of fusion? The heat of fusion definition communicates a form of energy that is either. Heat Fusion Definition.
From www.slideserve.com
PPT 4.3 SPECIFIC LATENT HEAT PowerPoint Presentation, free download Heat Fusion Definition What is heat of fusion? The heat of fusion (see thermal fusion), the heat that must be applied to melt a solid, must be removed from the liquid to freeze it. The concept of heat of fusion, often denoted by the symbol δh fus, is a critical parameter in thermodynamics and material. The heat of fusion definition communicates a form. Heat Fusion Definition.
From www.slideserve.com
PPT Change of State PowerPoint Presentation, free download ID1562618 Heat Fusion Definition The molar heat of fusion (δhfus) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to a liquid. The heat of fusion (see thermal fusion), the heat that must be applied to melt a solid, must be removed from the liquid to freeze it. What is heat of fusion?. Heat Fusion Definition.
From printablekopierdu.z21.web.core.windows.net
Heat Of Fusion Chemistry Heat Fusion Definition Some liquids can be supercooled—i.e., cooled below. The heat of fusion (see thermal fusion), the heat that must be applied to melt a solid, must be removed from the liquid to freeze it. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions. Heat Fusion Definition.
From www.slideserve.com
PPT 4.3 SPECIFIC LATENT HEAT PowerPoint Presentation, free download Heat Fusion Definition The molar heat of solidification (δhsolid). The heat of fusion is the amount of energy required to change a substance from a solid to a liquid at its melting point without. What is heat of fusion? The concept of heat of fusion, often denoted by the symbol δh fus, is a critical parameter in thermodynamics and material. Some liquids can. Heat Fusion Definition.
From ar.inspiredpencil.com
Heat And Fusion Definition Heat Fusion Definition Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant. The molar heat of fusion (δhfus) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to a liquid. Some liquids. Heat Fusion Definition.
From www.pw.live
Heat Of Fusion Formula Definition, Formula, Solved Questions Heat Fusion Definition The molar heat of solidification (δhsolid). Some liquids can be supercooled—i.e., cooled below. The molar heat of fusion (δhfus) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to a liquid. The concept of heat of fusion, often denoted by the symbol δh fus, is a critical parameter in. Heat Fusion Definition.
From www.slideshare.net
Energy ch 16 Heat Fusion Definition The molar heat of fusion (δhfus) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to a liquid. This process is better known as melting, or heat of fusion, and results in the molecules within the substance becoming less. The heat of fusion is the amount of energy required. Heat Fusion Definition.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk Heat Fusion Definition The molar heat of solidification (δhsolid). The heat of fusion definition communicates a form of energy that is either released or absorbed when a certain amount of matter is changing phase. The heat of fusion is the amount of energy required to change a substance from a solid to a liquid at its melting point without. This process is better. Heat Fusion Definition.
From www.slideserve.com
PPT Heat of Fusion PowerPoint Presentation, free download ID2249917 Heat Fusion Definition The heat of fusion (see thermal fusion), the heat that must be applied to melt a solid, must be removed from the liquid to freeze it. The molar heat of solidification (δhsolid). Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of. Heat Fusion Definition.
From definitionxc.blogspot.com
Molar Heat Of Fusion Definition Chemistry DEFINITIONXC Heat Fusion Definition The heat of fusion is alternatively known. The heat of fusion is the amount of energy required to change a substance from a solid to a liquid at its melting point without. What is heat of fusion? The concept of heat of fusion, often denoted by the symbol δh fus, is a critical parameter in thermodynamics and material. The molar. Heat Fusion Definition.
From www.ck12.org
Heat of fusion Overview ( Video ) Chemistry CK12 Foundation Heat Fusion Definition The heat of fusion (see thermal fusion), the heat that must be applied to melt a solid, must be removed from the liquid to freeze it. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant. What is heat of fusion?. Heat Fusion Definition.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk Heat Fusion Definition This process is better known as melting, or heat of fusion, and results in the molecules within the substance becoming less. The molar heat of fusion (δhfus) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to a liquid. What is heat of fusion? The heat of fusion is. Heat Fusion Definition.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID1828952 Heat Fusion Definition The heat of fusion is the amount of energy required to change a substance from a solid to a liquid at its melting point without. What is heat of fusion? This process is better known as melting, or heat of fusion, and results in the molecules within the substance becoming less. The concept of heat of fusion, often denoted by. Heat Fusion Definition.
From ar.inspiredpencil.com
Heat And Fusion Definition Heat Fusion Definition What is heat of fusion? The heat of fusion (see thermal fusion), the heat that must be applied to melt a solid, must be removed from the liquid to freeze it. The heat of fusion is alternatively known. Some liquids can be supercooled—i.e., cooled below. The heat of fusion definition communicates a form of energy that is either released or. Heat Fusion Definition.
From www.sliderbase.com
Heat of Fusion Heat Fusion Definition The molar heat of solidification (δhsolid). The heat of fusion definition communicates a form of energy that is either released or absorbed when a certain amount of matter is changing phase. The heat of fusion is alternatively known. This process is better known as melting, or heat of fusion, and results in the molecules within the substance becoming less. The. Heat Fusion Definition.
From www.slideserve.com
PPT Fundamentals of Heat Transfer PowerPoint Presentation, free Heat Fusion Definition The concept of heat of fusion, often denoted by the symbol δh fus, is a critical parameter in thermodynamics and material. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant. The heat of fusion (see thermal fusion), the heat that. Heat Fusion Definition.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk Heat Fusion Definition Some liquids can be supercooled—i.e., cooled below. The heat of fusion definition communicates a form of energy that is either released or absorbed when a certain amount of matter is changing phase. What is heat of fusion? Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze. Heat Fusion Definition.
From study.com
Heat of Fusion Definition, Equation & Examples Video & Lesson Heat Fusion Definition What is heat of fusion? The heat of fusion is the amount of energy required to change a substance from a solid to a liquid at its melting point without. The heat of fusion is alternatively known. The concept of heat of fusion, often denoted by the symbol δh fus, is a critical parameter in thermodynamics and material. The molar. Heat Fusion Definition.
From study.com
Heat of Fusion Definition, Formula & Vaporization Lesson Heat Fusion Definition This process is better known as melting, or heat of fusion, and results in the molecules within the substance becoming less. The heat of fusion is alternatively known. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant. Some liquids can. Heat Fusion Definition.
From ar.inspiredpencil.com
Heat And Fusion Definition Heat Fusion Definition The molar heat of solidification (δhsolid). The heat of fusion (see thermal fusion), the heat that must be applied to melt a solid, must be removed from the liquid to freeze it. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of. Heat Fusion Definition.
From www.slideserve.com
PPT Physical Behavior of Matter PowerPoint Presentation, free Heat Fusion Definition This process is better known as melting, or heat of fusion, and results in the molecules within the substance becoming less. The concept of heat of fusion, often denoted by the symbol δh fus, is a critical parameter in thermodynamics and material. What is heat of fusion? The molar heat of fusion (δhfus) of a substance is the heat absorbed. Heat Fusion Definition.
From gamesmartz.com
Heat Of Fusion Definition & Image GameSmartz Heat Fusion Definition The heat of fusion (see thermal fusion), the heat that must be applied to melt a solid, must be removed from the liquid to freeze it. What is heat of fusion? Some liquids can be supercooled—i.e., cooled below. The molar heat of solidification (δhsolid). The molar heat of fusion (δhfus) of a substance is the heat absorbed by one mole. Heat Fusion Definition.
From www.slideserve.com
PPT Ch. 3.1 Solids, Liquids, Gases PowerPoint Presentation, free Heat Fusion Definition What is heat of fusion? The concept of heat of fusion, often denoted by the symbol δh fus, is a critical parameter in thermodynamics and material. Some liquids can be supercooled—i.e., cooled below. The heat of fusion is the amount of energy required to change a substance from a solid to a liquid at its melting point without. The molar. Heat Fusion Definition.