Unsaturated Vs Dilute . A dilute solution is one. In other words, the solution is not saturated. An unsaturated solution is more dilute than a saturated solution. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. How can you tell if a solution is saturated or unsaturated? We will begin our discussion of solution concentration with two related and relative terms: All of the solute dissolves in the solvent. In chemistry, the term unsaturated usually refers to one of two things: If more solute is added and it does not dissolve, then the original solution was saturated. When referring to chemical solutions, an unsaturated solution is able to dissolve more solute. An unsaturated solution is a chemical solution in which the solute concentration is lower than its equilibrium solubility. Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to.
from laboratoryhub.com
If more solute is added and it does not dissolve, then the original solution was saturated. All of the solute dissolves in the solvent. An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. In chemistry, the term unsaturated usually refers to one of two things: How can you tell if a solution is saturated or unsaturated? Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. An unsaturated solution is more dilute than a saturated solution. An unsaturated solution is a chemical solution in which the solute concentration is lower than its equilibrium solubility. When referring to chemical solutions, an unsaturated solution is able to dissolve more solute. In other words, the solution is not saturated.
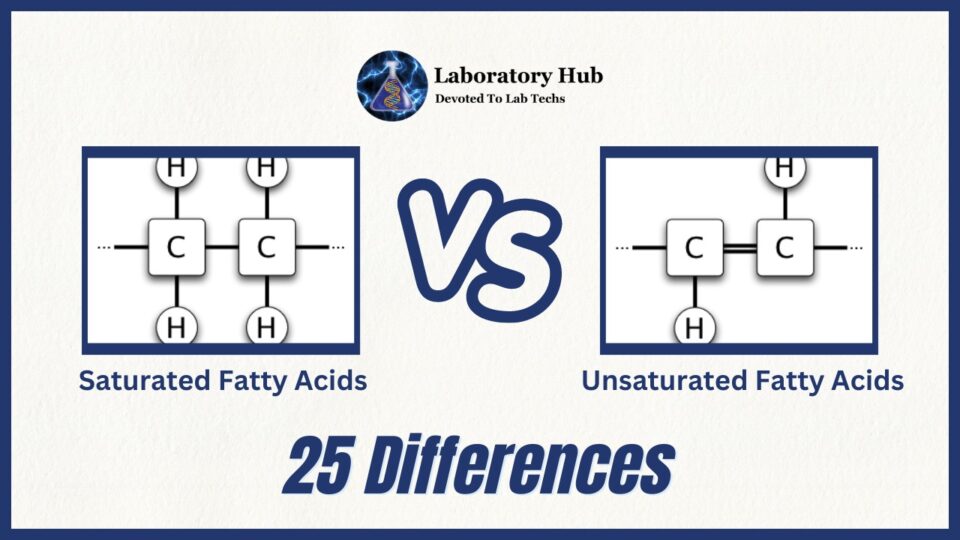
Saturated vs Unsaturated fatty acids 25 Differences
Unsaturated Vs Dilute How can you tell if a solution is saturated or unsaturated? Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to. In chemistry, the term unsaturated usually refers to one of two things: How can you tell if a solution is saturated or unsaturated? If more solute is added and it does not dissolve, then the original solution was saturated. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. An unsaturated solution is a chemical solution in which the solute concentration is lower than its equilibrium solubility. When referring to chemical solutions, an unsaturated solution is able to dissolve more solute. All of the solute dissolves in the solvent. A dilute solution is one. An unsaturated solution is more dilute than a saturated solution. An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. We will begin our discussion of solution concentration with two related and relative terms: In other words, the solution is not saturated.
From sciencenotes.org
Supersaturated Solution Definition and Examples Unsaturated Vs Dilute A dilute solution is one. Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. How can you tell if a solution is saturated or unsaturated? If more solute. Unsaturated Vs Dilute.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID6553222 Unsaturated Vs Dilute How can you tell if a solution is saturated or unsaturated? When referring to chemical solutions, an unsaturated solution is able to dissolve more solute. In chemistry, the term unsaturated usually refers to one of two things: Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. In other. Unsaturated Vs Dilute.
From www.3ditalian.com
What is the Difference Between Dilute and Unsaturated Solution Unsaturated Vs Dilute An unsaturated solution is a chemical solution in which the solute concentration is lower than its equilibrium solubility. A dilute solution is one. All of the solute dissolves in the solvent. We will begin our discussion of solution concentration with two related and relative terms: If more solute is added and it does not dissolve, then the original solution was. Unsaturated Vs Dilute.
From www.youtube.com
Is matter around us pure 03 Saturated vs unsaturated solution Unsaturated Vs Dilute How can you tell if a solution is saturated or unsaturated? A dilute solution is one. An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. If more solute is added and it does not dissolve, then the original solution was saturated. Apply a solubility conversion factor to calculate. Unsaturated Vs Dilute.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID252347 Unsaturated Vs Dilute An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. In other words, the solution is not saturated. A dilute solution is one. How can you tell if a solution is saturated or unsaturated? If more solute is added and it does not dissolve, then the original solution was. Unsaturated Vs Dilute.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download Unsaturated Vs Dilute We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is one. All of the solute dissolves in the solvent. When referring to chemical solutions, an unsaturated solution is able to dissolve more solute. In chemistry, the term unsaturated usually refers to one of two things: An unsaturated solution is a solution that. Unsaturated Vs Dilute.
From www.slideserve.com
PPT Lipids PowerPoint Presentation, free download ID6619297 Unsaturated Vs Dilute In chemistry, the term unsaturated usually refers to one of two things: An unsaturated solution is a chemical solution in which the solute concentration is lower than its equilibrium solubility. We will begin our discussion of solution concentration with two related and relative terms: An unsaturated solution is more dilute than a saturated solution. In other words, the solution is. Unsaturated Vs Dilute.
From www.slideserve.com
PPT Grade 7 Science PowerPoint Presentation, free download ID2229802 Unsaturated Vs Dilute In chemistry, the term unsaturated usually refers to one of two things: An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. When referring to chemical solutions, an unsaturated solution is able to dissolve more solute. All of the solute dissolves in the solvent. Dilute solutions have a lower. Unsaturated Vs Dilute.
From solanolabs.com
Saturated solution definition chemistry Difference Between Saturated Unsaturated Vs Dilute All of the solute dissolves in the solvent. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. When referring to chemical solutions, an unsaturated solution is able to dissolve more solute. In chemistry, the term unsaturated usually refers to one of two things: How can you tell if. Unsaturated Vs Dilute.
From sciencenotes.org
Unsaturated Solution Definition and Examples in Chemistry Unsaturated Vs Dilute How can you tell if a solution is saturated or unsaturated? In other words, the solution is not saturated. An unsaturated solution is more dilute than a saturated solution. An unsaturated solution is a chemical solution in which the solute concentration is lower than its equilibrium solubility. Dilute solutions have a lower density and lighter color compared to concentrated solutions,. Unsaturated Vs Dilute.
From www.slideserve.com
PPT Unit 6 Solutions PowerPoint Presentation, free download ID5505928 Unsaturated Vs Dilute How can you tell if a solution is saturated or unsaturated? A dilute solution is one. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Dilute solutions. Unsaturated Vs Dilute.
From www.youtube.com
Saturated Unsaturated and Supersaturated Solutions What is the Unsaturated Vs Dilute Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. All of the solute dissolves in the solvent. An unsaturated solution is more dilute than a saturated solution. How. Unsaturated Vs Dilute.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Unsaturated Vs Dilute We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is one. If more solute is added and it does not dissolve, then the original solution was saturated. In chemistry, the term unsaturated usually refers to one of two things: Dilute solutions have a lower density and lighter color compared to concentrated solutions,. Unsaturated Vs Dilute.
From laboratoryhub.com
Saturated vs Unsaturated fatty acids 25 Differences Unsaturated Vs Dilute We will begin our discussion of solution concentration with two related and relative terms: An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. In other words, the solution is not saturated. In chemistry, the term unsaturated usually refers to one of two things: How can you tell if. Unsaturated Vs Dilute.
From www.youtube.com
Solutions Lesson 1 Solutions and Solubility YouTube Unsaturated Vs Dilute How can you tell if a solution is saturated or unsaturated? An unsaturated solution is more dilute than a saturated solution. A dilute solution is one. We will begin our discussion of solution concentration with two related and relative terms: An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being. Unsaturated Vs Dilute.
From dokumen.tips
(PPT) Notes on Solutions. Qualitative ways of describing solutions Unsaturated Vs Dilute An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to. When referring to chemical solutions, an unsaturated solution is able to dissolve more solute. We will begin our discussion. Unsaturated Vs Dilute.
From www.slideserve.com
PPT Ch 15/16 Water and its Properties. Test Review PowerPoint Unsaturated Vs Dilute In other words, the solution is not saturated. An unsaturated solution is a chemical solution in which the solute concentration is lower than its equilibrium solubility. How can you tell if a solution is saturated or unsaturated? In chemistry, the term unsaturated usually refers to one of two things: An unsaturated solution is a solution that contains less than the. Unsaturated Vs Dilute.
From ar.inspiredpencil.com
Types Of Solutions Saturated Unsaturated And Supersaturated Unsaturated Vs Dilute Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. A dilute solution is one. An unsaturated solution is a chemical solution in which the solute concentration is lower than its equilibrium solubility. All of the solute dissolves in the solvent. An unsaturated solution is more dilute than a. Unsaturated Vs Dilute.
From www.numerade.com
SOLVED 'Using the triple venn diagram below compare and contrast the Unsaturated Vs Dilute How can you tell if a solution is saturated or unsaturated? Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to. An unsaturated solution is more dilute than a saturated solution. A dilute solution is one. In chemistry, the term unsaturated usually refers to one of two things: All of. Unsaturated Vs Dilute.
From www.slideshare.net
Chemistry Chp 16 Solutions PowerPoint (shortened) Unsaturated Vs Dilute If more solute is added and it does not dissolve, then the original solution was saturated. In chemistry, the term unsaturated usually refers to one of two things: We will begin our discussion of solution concentration with two related and relative terms: Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the. Unsaturated Vs Dilute.
From www.numerade.com
SOLVED A solution can be both dilute and concentrated saturated und Unsaturated Vs Dilute All of the solute dissolves in the solvent. In other words, the solution is not saturated. Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to. An unsaturated solution is more dilute than a saturated solution. In chemistry, the term unsaturated usually refers to one of two things: An unsaturated. Unsaturated Vs Dilute.
From www.ck12.org
Saturation CK12 Foundation Unsaturated Vs Dilute In chemistry, the term unsaturated usually refers to one of two things: If more solute is added and it does not dissolve, then the original solution was saturated. An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. In other words, the solution is not saturated. When referring to. Unsaturated Vs Dilute.
From www.youtube.com
Saturated vs Unsaturated fatty acids Definition, 20 Differences Unsaturated Vs Dilute An unsaturated solution is a chemical solution in which the solute concentration is lower than its equilibrium solubility. In chemistry, the term unsaturated usually refers to one of two things: An unsaturated solution is more dilute than a saturated solution. When referring to chemical solutions, an unsaturated solution is able to dissolve more solute. Apply a solubility conversion factor to. Unsaturated Vs Dilute.
From www.slideserve.com
PPT Chapter 1516 study guide PowerPoint Presentation, free download Unsaturated Vs Dilute When referring to chemical solutions, an unsaturated solution is able to dissolve more solute. An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. All of the solute dissolves in the solvent. An unsaturated solution is a chemical solution in which the solute concentration is lower than its equilibrium. Unsaturated Vs Dilute.
From slideplayer.com
Water and Aqueous Systems ppt download Unsaturated Vs Dilute If more solute is added and it does not dissolve, then the original solution was saturated. An unsaturated solution is a chemical solution in which the solute concentration is lower than its equilibrium solubility. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. Dilute solutions have a lower. Unsaturated Vs Dilute.
From www.teachoo.com
Difference between Saturated and Unsaturated Solution Teachoo Unsaturated Vs Dilute If more solute is added and it does not dissolve, then the original solution was saturated. Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. All of the. Unsaturated Vs Dilute.
From www.pinterest.com
saturated unsaturated and supersaturated solutions Google Search Unsaturated Vs Dilute An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. A dilute solution is one. When referring to chemical solutions, an unsaturated solution is able to dissolve more solute. We will begin our discussion of solution concentration with two related and relative terms: How can you tell if a. Unsaturated Vs Dilute.
From www.youtube.com
CONCENTRATION OF SOLUTIONS SATURATED & UNSATURATED DILUTE Unsaturated Vs Dilute A dilute solution is one. When referring to chemical solutions, an unsaturated solution is able to dissolve more solute. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. An unsaturated solution is a chemical solution in which the solute concentration is lower than its equilibrium solubility. How can. Unsaturated Vs Dilute.
From www.teachoo.com
Classification of Acids on Basis of source, Concentration Teachoo Unsaturated Vs Dilute An unsaturated solution is more dilute than a saturated solution. In chemistry, the term unsaturated usually refers to one of two things: In other words, the solution is not saturated. We will begin our discussion of solution concentration with two related and relative terms: If more solute is added and it does not dissolve, then the original solution was saturated.. Unsaturated Vs Dilute.
From www.yaclass.in
Types of solutions Based on the amount of the solute — lesson. Science Unsaturated Vs Dilute Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to. In chemistry, the term unsaturated usually refers to one of two things: We will begin our discussion of solution concentration with two related and relative terms: In other words, the solution is not saturated. An unsaturated solution is a solution. Unsaturated Vs Dilute.
From quizlet.com
Mixtures and Solutions Diagram Quizlet Unsaturated Vs Dilute Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. How can you tell if a solution is saturated or unsaturated? We will begin our discussion of solution concentration with two related and relative terms: Dilute solutions have a lower density and lighter color compared to concentrated solutions, while. Unsaturated Vs Dilute.
From slideplayer.com
Chemistry Unit Benchmark ppt download Unsaturated Vs Dilute A dilute solution is one. An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. When referring to chemical solutions, an unsaturated solution is able to dissolve more solute. We will begin our discussion of solution concentration with two related and relative terms: In chemistry, the term unsaturated usually. Unsaturated Vs Dilute.
From www.youtube.com
Dilute & Concentrated solution Saturated & Unsaturated solution Class Unsaturated Vs Dilute We will begin our discussion of solution concentration with two related and relative terms: An unsaturated solution is more dilute than a saturated solution. An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. An unsaturated solution is a chemical solution in which the solute concentration is lower than. Unsaturated Vs Dilute.
From www.slideserve.com
PPT What is osmosis? PowerPoint Presentation, free download ID2712925 Unsaturated Vs Dilute In chemistry, the term unsaturated usually refers to one of two things: When referring to chemical solutions, an unsaturated solution is able to dissolve more solute. An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. A dilute solution is one. In other words, the solution is not saturated.. Unsaturated Vs Dilute.
From examples.yourdictionary.com
Examples of Saturated Solution YourDictionary Unsaturated Vs Dilute A dilute solution is one. If more solute is added and it does not dissolve, then the original solution was saturated. All of the solute dissolves in the solvent. An unsaturated solution is a chemical solution in which the solute concentration is lower than its equilibrium solubility. In chemistry, the term unsaturated usually refers to one of two things: Dilute. Unsaturated Vs Dilute.