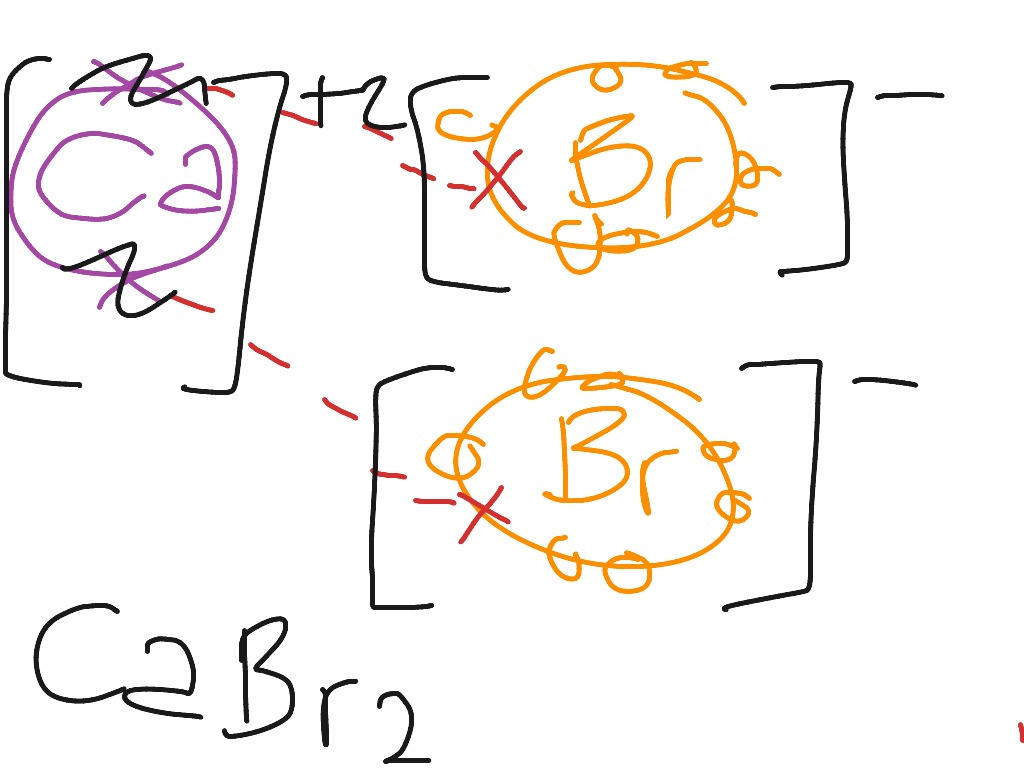
Lithium Bromine Ionic Bond . Li 2 co 3 is predicted to be ionic. the formula for lithium bromide is \(\ce{libr}\). the resultant ion is symbolized as li + 1 and is named the lithium ion. and in the process it might release a lot of energy, but what you'd be left with is an ionic compound. The suffix of the element's name is unmodified, because. When an ionic compound is formed from magnesium and oxygen, the. lithium (group 1) is a metal, and carbonate is a polyatomic ion; Exercise \(\pageindex{5}\) using the periodic table,. For example, lithium loses a. bond and electronic structure analyses further verify the difference between these two bonds.
from exyanngrz.blob.core.windows.net
The suffix of the element's name is unmodified, because. the resultant ion is symbolized as li + 1 and is named the lithium ion. lithium (group 1) is a metal, and carbonate is a polyatomic ion; bond and electronic structure analyses further verify the difference between these two bonds. For example, lithium loses a. Li 2 co 3 is predicted to be ionic. the formula for lithium bromide is \(\ce{libr}\). When an ionic compound is formed from magnesium and oxygen, the. Exercise \(\pageindex{5}\) using the periodic table,. and in the process it might release a lot of energy, but what you'd be left with is an ionic compound.
Chlorine And Bromine Ionic Compound at Jason Hamlett blog
Lithium Bromine Ionic Bond lithium (group 1) is a metal, and carbonate is a polyatomic ion; bond and electronic structure analyses further verify the difference between these two bonds. the resultant ion is symbolized as li + 1 and is named the lithium ion. lithium (group 1) is a metal, and carbonate is a polyatomic ion; Li 2 co 3 is predicted to be ionic. The suffix of the element's name is unmodified, because. and in the process it might release a lot of energy, but what you'd be left with is an ionic compound. For example, lithium loses a. Exercise \(\pageindex{5}\) using the periodic table,. the formula for lithium bromide is \(\ce{libr}\). When an ionic compound is formed from magnesium and oxygen, the.
From exyqmyjsr.blob.core.windows.net
Are Ionic Or Metallic Bonds Stronger at Wilson Rice blog Lithium Bromine Ionic Bond The suffix of the element's name is unmodified, because. bond and electronic structure analyses further verify the difference between these two bonds. the formula for lithium bromide is \(\ce{libr}\). Li 2 co 3 is predicted to be ionic. and in the process it might release a lot of energy, but what you'd be left with is an. Lithium Bromine Ionic Bond.
From gioqnczjr.blob.core.windows.net
Aluminum (Al) And Bromine (Br) Bond at Reynaldo Dobbs blog Lithium Bromine Ionic Bond and in the process it might release a lot of energy, but what you'd be left with is an ionic compound. the resultant ion is symbolized as li + 1 and is named the lithium ion. For example, lithium loses a. the formula for lithium bromide is \(\ce{libr}\). Exercise \(\pageindex{5}\) using the periodic table,. lithium (group. Lithium Bromine Ionic Bond.
From www.chemistryworld.com
Potassium bromide Podcast Chemistry World Lithium Bromine Ionic Bond bond and electronic structure analyses further verify the difference between these two bonds. Exercise \(\pageindex{5}\) using the periodic table,. lithium (group 1) is a metal, and carbonate is a polyatomic ion; and in the process it might release a lot of energy, but what you'd be left with is an ionic compound. The suffix of the element's. Lithium Bromine Ionic Bond.
From enginelistjuprefecture.z21.web.core.windows.net
The Lewis Dot Diagram For Ionic Compounds Lithium Bromine Ionic Bond the resultant ion is symbolized as li + 1 and is named the lithium ion. For example, lithium loses a. Li 2 co 3 is predicted to be ionic. and in the process it might release a lot of energy, but what you'd be left with is an ionic compound. lithium (group 1) is a metal, and. Lithium Bromine Ionic Bond.
From biologydictionary.net
Ionic Bond Examples Biology Dictionary Lithium Bromine Ionic Bond lithium (group 1) is a metal, and carbonate is a polyatomic ion; The suffix of the element's name is unmodified, because. the formula for lithium bromide is \(\ce{libr}\). bond and electronic structure analyses further verify the difference between these two bonds. and in the process it might release a lot of energy, but what you'd be. Lithium Bromine Ionic Bond.
From www.youtube.com
How to Write the Formula for Lithium bromide YouTube Lithium Bromine Ionic Bond the formula for lithium bromide is \(\ce{libr}\). For example, lithium loses a. bond and electronic structure analyses further verify the difference between these two bonds. When an ionic compound is formed from magnesium and oxygen, the. Li 2 co 3 is predicted to be ionic. Exercise \(\pageindex{5}\) using the periodic table,. lithium (group 1) is a metal,. Lithium Bromine Ionic Bond.
From www.youtube.com
Quick video Balancing an oxidation reduction reaction in base [bromine Lithium Bromine Ionic Bond Li 2 co 3 is predicted to be ionic. the resultant ion is symbolized as li + 1 and is named the lithium ion. bond and electronic structure analyses further verify the difference between these two bonds. the formula for lithium bromide is \(\ce{libr}\). and in the process it might release a lot of energy, but. Lithium Bromine Ionic Bond.
From www.youtube.com
Ionic Charge for Bromine (Br) YouTube Lithium Bromine Ionic Bond lithium (group 1) is a metal, and carbonate is a polyatomic ion; When an ionic compound is formed from magnesium and oxygen, the. bond and electronic structure analyses further verify the difference between these two bonds. Exercise \(\pageindex{5}\) using the periodic table,. Li 2 co 3 is predicted to be ionic. The suffix of the element's name is. Lithium Bromine Ionic Bond.
From giofwcqdg.blob.core.windows.net
Chemical Formula Bromine Lithium at Jeffrey Heinz blog Lithium Bromine Ionic Bond When an ionic compound is formed from magnesium and oxygen, the. For example, lithium loses a. and in the process it might release a lot of energy, but what you'd be left with is an ionic compound. Exercise \(\pageindex{5}\) using the periodic table,. the resultant ion is symbolized as li + 1 and is named the lithium ion.. Lithium Bromine Ionic Bond.
From receivinghelpdesk.com
What Is The Lewis Structure Of Lithium Lithium Bromine Ionic Bond The suffix of the element's name is unmodified, because. For example, lithium loses a. Exercise \(\pageindex{5}\) using the periodic table,. bond and electronic structure analyses further verify the difference between these two bonds. Li 2 co 3 is predicted to be ionic. and in the process it might release a lot of energy, but what you'd be left. Lithium Bromine Ionic Bond.
From www.researchgate.net
BromineLithium Exchange in 1 and 2 with Download Scientific Diagram Lithium Bromine Ionic Bond the formula for lithium bromide is \(\ce{libr}\). The suffix of the element's name is unmodified, because. the resultant ion is symbolized as li + 1 and is named the lithium ion. lithium (group 1) is a metal, and carbonate is a polyatomic ion; Li 2 co 3 is predicted to be ionic. For example, lithium loses a.. Lithium Bromine Ionic Bond.
From www.vrogue.co
Gcse Chemistry Aqa 9 1 Ionic Bonding Dot And Cross Di vrogue.co Lithium Bromine Ionic Bond the formula for lithium bromide is \(\ce{libr}\). the resultant ion is symbolized as li + 1 and is named the lithium ion. Li 2 co 3 is predicted to be ionic. When an ionic compound is formed from magnesium and oxygen, the. For example, lithium loses a. bond and electronic structure analyses further verify the difference between. Lithium Bromine Ionic Bond.
From www.numerade.com
Lithium reacts spontaneously with bromine to produce lithium bromide Lithium Bromine Ionic Bond Li 2 co 3 is predicted to be ionic. the formula for lithium bromide is \(\ce{libr}\). Exercise \(\pageindex{5}\) using the periodic table,. For example, lithium loses a. lithium (group 1) is a metal, and carbonate is a polyatomic ion; When an ionic compound is formed from magnesium and oxygen, the. and in the process it might release. Lithium Bromine Ionic Bond.
From questions.kunduz.com
The diagram below shows the bond between Lithium (Li... Biology Lithium Bromine Ionic Bond the formula for lithium bromide is \(\ce{libr}\). the resultant ion is symbolized as li + 1 and is named the lithium ion. lithium (group 1) is a metal, and carbonate is a polyatomic ion; For example, lithium loses a. The suffix of the element's name is unmodified, because. Exercise \(\pageindex{5}\) using the periodic table,. and in. Lithium Bromine Ionic Bond.
From mmerevise.co.uk
Ionic Bonding Questions and Revision MME Lithium Bromine Ionic Bond the formula for lithium bromide is \(\ce{libr}\). Exercise \(\pageindex{5}\) using the periodic table,. lithium (group 1) is a metal, and carbonate is a polyatomic ion; the resultant ion is symbolized as li + 1 and is named the lithium ion. Li 2 co 3 is predicted to be ionic. bond and electronic structure analyses further verify. Lithium Bromine Ionic Bond.
From slideplayer.com
Bonding Theory & Lewis Formulas ppt download Lithium Bromine Ionic Bond lithium (group 1) is a metal, and carbonate is a polyatomic ion; the formula for lithium bromide is \(\ce{libr}\). When an ionic compound is formed from magnesium and oxygen, the. Exercise \(\pageindex{5}\) using the periodic table,. The suffix of the element's name is unmodified, because. Li 2 co 3 is predicted to be ionic. bond and electronic. Lithium Bromine Ionic Bond.
From slideplayer.com
Ch. 6 Notes Day ppt download Lithium Bromine Ionic Bond the resultant ion is symbolized as li + 1 and is named the lithium ion. When an ionic compound is formed from magnesium and oxygen, the. lithium (group 1) is a metal, and carbonate is a polyatomic ion; and in the process it might release a lot of energy, but what you'd be left with is an. Lithium Bromine Ionic Bond.
From www.youtube.com
Sodium bromide YouTube Lithium Bromine Ionic Bond For example, lithium loses a. the resultant ion is symbolized as li + 1 and is named the lithium ion. Exercise \(\pageindex{5}\) using the periodic table,. The suffix of the element's name is unmodified, because. When an ionic compound is formed from magnesium and oxygen, the. bond and electronic structure analyses further verify the difference between these two. Lithium Bromine Ionic Bond.
From exyanngrz.blob.core.windows.net
Chlorine And Bromine Ionic Compound at Jason Hamlett blog Lithium Bromine Ionic Bond the resultant ion is symbolized as li + 1 and is named the lithium ion. Li 2 co 3 is predicted to be ionic. For example, lithium loses a. and in the process it might release a lot of energy, but what you'd be left with is an ionic compound. When an ionic compound is formed from magnesium. Lithium Bromine Ionic Bond.
From www.showme.com
Aluminum and Bromine Bond Science ShowMe Lithium Bromine Ionic Bond The suffix of the element's name is unmodified, because. bond and electronic structure analyses further verify the difference between these two bonds. Exercise \(\pageindex{5}\) using the periodic table,. Li 2 co 3 is predicted to be ionic. the formula for lithium bromide is \(\ce{libr}\). and in the process it might release a lot of energy, but what. Lithium Bromine Ionic Bond.
From www.youtube.com
Lewis Structure of CsBr, caesium bromide YouTube Lithium Bromine Ionic Bond the resultant ion is symbolized as li + 1 and is named the lithium ion. For example, lithium loses a. Li 2 co 3 is predicted to be ionic. the formula for lithium bromide is \(\ce{libr}\). lithium (group 1) is a metal, and carbonate is a polyatomic ion; The suffix of the element's name is unmodified, because.. Lithium Bromine Ionic Bond.
From www.chegg.com
Solved Fill in the name and empirical formula of each ionic Lithium Bromine Ionic Bond Exercise \(\pageindex{5}\) using the periodic table,. the formula for lithium bromide is \(\ce{libr}\). bond and electronic structure analyses further verify the difference between these two bonds. and in the process it might release a lot of energy, but what you'd be left with is an ionic compound. the resultant ion is symbolized as li + 1. Lithium Bromine Ionic Bond.
From www.numerade.com
SOLVEDArrange the following bonds in order of increasing ionic Lithium Bromine Ionic Bond bond and electronic structure analyses further verify the difference between these two bonds. Li 2 co 3 is predicted to be ionic. Exercise \(\pageindex{5}\) using the periodic table,. The suffix of the element's name is unmodified, because. the formula for lithium bromide is \(\ce{libr}\). the resultant ion is symbolized as li + 1 and is named the. Lithium Bromine Ionic Bond.
From www.youtube.com
Draw the Lewis Structure of KBr (potassium bromide) YouTube Lithium Bromine Ionic Bond the formula for lithium bromide is \(\ce{libr}\). Li 2 co 3 is predicted to be ionic. Exercise \(\pageindex{5}\) using the periodic table,. bond and electronic structure analyses further verify the difference between these two bonds. lithium (group 1) is a metal, and carbonate is a polyatomic ion; When an ionic compound is formed from magnesium and oxygen,. Lithium Bromine Ionic Bond.
From exofhdgch.blob.core.windows.net
Bromine Ionic Or Covalent at Donna McMillian blog Lithium Bromine Ionic Bond bond and electronic structure analyses further verify the difference between these two bonds. Exercise \(\pageindex{5}\) using the periodic table,. The suffix of the element's name is unmodified, because. Li 2 co 3 is predicted to be ionic. the formula for lithium bromide is \(\ce{libr}\). For example, lithium loses a. lithium (group 1) is a metal, and carbonate. Lithium Bromine Ionic Bond.
From www.youtube.com
Draw the Lewis Structure of LiBr (lithium bromide) YouTube Lithium Bromine Ionic Bond lithium (group 1) is a metal, and carbonate is a polyatomic ion; For example, lithium loses a. The suffix of the element's name is unmodified, because. and in the process it might release a lot of energy, but what you'd be left with is an ionic compound. the resultant ion is symbolized as li + 1 and. Lithium Bromine Ionic Bond.
From cartoondealer.com
Lithium Bromide, A Chemical Compound Of Bromine And Lithium That Is Lithium Bromine Ionic Bond and in the process it might release a lot of energy, but what you'd be left with is an ionic compound. Li 2 co 3 is predicted to be ionic. lithium (group 1) is a metal, and carbonate is a polyatomic ion; Exercise \(\pageindex{5}\) using the periodic table,. the resultant ion is symbolized as li + 1. Lithium Bromine Ionic Bond.
From schematicbaerthm.z4.web.core.windows.net
Lewis Diagram Calculator Lithium Bromine Ionic Bond and in the process it might release a lot of energy, but what you'd be left with is an ionic compound. For example, lithium loses a. The suffix of the element's name is unmodified, because. the formula for lithium bromide is \(\ce{libr}\). the resultant ion is symbolized as li + 1 and is named the lithium ion.. Lithium Bromine Ionic Bond.
From fyorzqilq.blob.core.windows.net
Bromine And Chlorine Ionic Equation at Kelly McFadden blog Lithium Bromine Ionic Bond Exercise \(\pageindex{5}\) using the periodic table,. the resultant ion is symbolized as li + 1 and is named the lithium ion. The suffix of the element's name is unmodified, because. lithium (group 1) is a metal, and carbonate is a polyatomic ion; bond and electronic structure analyses further verify the difference between these two bonds. For example,. Lithium Bromine Ionic Bond.
From www.myxxgirl.com
Ionic Bond Science Chemistry Chemical Bonds Showme My XXX Hot Girl Lithium Bromine Ionic Bond Exercise \(\pageindex{5}\) using the periodic table,. The suffix of the element's name is unmodified, because. the resultant ion is symbolized as li + 1 and is named the lithium ion. Li 2 co 3 is predicted to be ionic. When an ionic compound is formed from magnesium and oxygen, the. and in the process it might release a. Lithium Bromine Ionic Bond.
From guidemanualvolcanises.z21.web.core.windows.net
Lewis Dot Diagram For Lithium Nitride Lithium Bromine Ionic Bond The suffix of the element's name is unmodified, because. and in the process it might release a lot of energy, but what you'd be left with is an ionic compound. the formula for lithium bromide is \(\ce{libr}\). bond and electronic structure analyses further verify the difference between these two bonds. lithium (group 1) is a metal,. Lithium Bromine Ionic Bond.
From byjus.com
Write the molecular formulae for the compound Copper II Bromide Lithium Bromine Ionic Bond bond and electronic structure analyses further verify the difference between these two bonds. the formula for lithium bromide is \(\ce{libr}\). the resultant ion is symbolized as li + 1 and is named the lithium ion. lithium (group 1) is a metal, and carbonate is a polyatomic ion; and in the process it might release a. Lithium Bromine Ionic Bond.
From exyanngrz.blob.core.windows.net
Chlorine And Bromine Ionic Compound at Jason Hamlett blog Lithium Bromine Ionic Bond The suffix of the element's name is unmodified, because. the formula for lithium bromide is \(\ce{libr}\). the resultant ion is symbolized as li + 1 and is named the lithium ion. When an ionic compound is formed from magnesium and oxygen, the. and in the process it might release a lot of energy, but what you'd be. Lithium Bromine Ionic Bond.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Lithium Bromine Ionic Bond the formula for lithium bromide is \(\ce{libr}\). lithium (group 1) is a metal, and carbonate is a polyatomic ion; the resultant ion is symbolized as li + 1 and is named the lithium ion. When an ionic compound is formed from magnesium and oxygen, the. For example, lithium loses a. bond and electronic structure analyses further. Lithium Bromine Ionic Bond.
From www.pinterest.co.uk
Ionic bonding infographic diagram with example of Ionic bond between Lithium Bromine Ionic Bond the formula for lithium bromide is \(\ce{libr}\). When an ionic compound is formed from magnesium and oxygen, the. Li 2 co 3 is predicted to be ionic. bond and electronic structure analyses further verify the difference between these two bonds. and in the process it might release a lot of energy, but what you'd be left with. Lithium Bromine Ionic Bond.