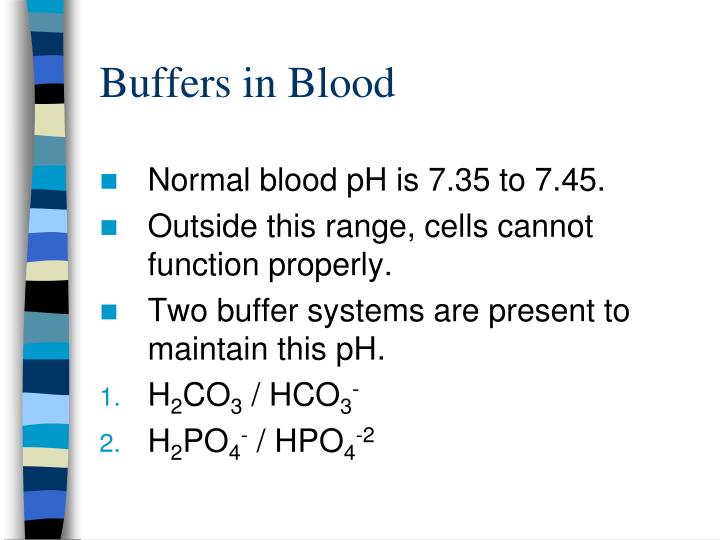
What Are The Major Buffer In The Blood . One mechanism the body uses to control blood ph involves the release of. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in. The ph of the blood is maintained between 7.35 and 7.45 by an. a buffer is a solution that resists sudden changes in ph. describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g.,. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. This is responsible for about 80% of extracellular.
from www.slideserve.com
describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g.,. One mechanism the body uses to control blood ph involves the release of. The ph of the blood is maintained between 7.35 and 7.45 by an. a buffer is a solution that resists sudden changes in ph. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. This is responsible for about 80% of extracellular.
PPT Chapter 8 Acids & Bases PowerPoint Presentation ID1121468
What Are The Major Buffer In The Blood One mechanism the body uses to control blood ph involves the release of. a buffer is a solution that resists sudden changes in ph. This is responsible for about 80% of extracellular. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in. describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g.,. One mechanism the body uses to control blood ph involves the release of. The ph of the blood is maintained between 7.35 and 7.45 by an. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.
From www.slideserve.com
PPT AcidBase Reactions Ch. 15 PowerPoint Presentation, free download What Are The Major Buffer In The Blood The ph of the blood is maintained between 7.35 and 7.45 by an. One mechanism the body uses to control blood ph involves the release of. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and. What Are The Major Buffer In The Blood.
From www.slideserve.com
PPT General, Organic, and Biochemistry, 7e PowerPoint Presentation What Are The Major Buffer In The Blood the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. a buffer is a solution that resists sudden changes in ph. This is responsible for about 80% of extracellular. describe the major buffer systems of. What Are The Major Buffer In The Blood.
From rowangroboone.blogspot.com
Certain Proteins Act as Buffers in the Blood RowangroBoone What Are The Major Buffer In The Blood One mechanism the body uses to control blood ph involves the release of. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in. This is responsible for about 80% of extracellular. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. The ph of. What Are The Major Buffer In The Blood.
From www.slideserve.com
PPT AcidBase Balance I. PowerPoint Presentation, free download ID What Are The Major Buffer In The Blood Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in. This is responsible for about 80% of extracellular. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The ph of the blood is maintained between 7.35 and 7.45 by an.. What Are The Major Buffer In The Blood.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation What Are The Major Buffer In The Blood One mechanism the body uses to control blood ph involves the release of. This is responsible for about 80% of extracellular. describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g.,. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. . What Are The Major Buffer In The Blood.
From poorvi77.blogspot.com
Poorveez ParadyzMedimuseion major buffer systems in the body What Are The Major Buffer In The Blood the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. a buffer is a solution that resists sudden changes in ph. The ph of the blood is maintained between 7.35 and 7.45 by an. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood. What Are The Major Buffer In The Blood.
From www.youtube.com
Blood Buffer Summary YouTube What Are The Major Buffer In The Blood a buffer is a solution that resists sudden changes in ph. The ph of the blood is maintained between 7.35 and 7.45 by an. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood. What Are The Major Buffer In The Blood.
From www.slideserve.com
PPT Chapter 8 Acids & Bases PowerPoint Presentation ID1121468 What Are The Major Buffer In The Blood Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. This is responsible for about 80%. What Are The Major Buffer In The Blood.
From www.slideserve.com
PPT Blood Buffers PowerPoint Presentation, free download ID2730981 What Are The Major Buffer In The Blood the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The ph of the blood is maintained between 7.35 and 7.45 by an. This is responsible for about 80% of extracellular. describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g.,. . What Are The Major Buffer In The Blood.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation What Are The Major Buffer In The Blood a buffer is a solution that resists sudden changes in ph. One mechanism the body uses to control blood ph involves the release of. The ph of the blood is maintained between 7.35 and 7.45 by an. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood contains a. What Are The Major Buffer In The Blood.
From www.slideserve.com
PPT Physiological system of blood. Functional importance of blood What Are The Major Buffer In The Blood describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g.,. The ph of the blood is maintained between 7.35 and 7.45 by an. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in. the buffer systems functioning in. What Are The Major Buffer In The Blood.
From dxopbwbtw.blob.core.windows.net
How Do Buffers Work In Blood at Carter Nolan blog What Are The Major Buffer In The Blood One mechanism the body uses to control blood ph involves the release of. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. This is responsible for about 80% of extracellular. The ph of the blood is maintained between 7.35 and 7.45 by an. describe the major buffer systems of the. What Are The Major Buffer In The Blood.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 What Are The Major Buffer In The Blood a buffer is a solution that resists sudden changes in ph. The ph of the blood is maintained between 7.35 and 7.45 by an. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −). What Are The Major Buffer In The Blood.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation What Are The Major Buffer In The Blood the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. The ph of the blood is maintained between 7.35 and 7.45 by an. One mechanism the body uses to control blood ph involves the release of. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3. What Are The Major Buffer In The Blood.
From www.slideshare.net
Buffer in the blood What Are The Major Buffer In The Blood The ph of the blood is maintained between 7.35 and 7.45 by an. describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g.,. One mechanism the body uses to control blood ph involves the release of. a buffer is a solution that resists sudden changes in ph. This is. What Are The Major Buffer In The Blood.
From www.slideserve.com
PPT MLAB 2401 Clinical Chemistry Keri BrophyMartinez PowerPoint What Are The Major Buffer In The Blood the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. This is responsible for about 80% of extracellular. The ph of the blood is maintained between 7.35 and 7.45 by an. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. describe the major buffer systems. What Are The Major Buffer In The Blood.
From www.slideserve.com
PPT RESPIRATORY SYSTEM 3 PowerPoint Presentation, free download What Are The Major Buffer In The Blood describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g.,. One mechanism the body uses to control blood ph involves the release of. This is responsible for about 80% of extracellular. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human. What Are The Major Buffer In The Blood.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation What Are The Major Buffer In The Blood The ph of the blood is maintained between 7.35 and 7.45 by an. a buffer is a solution that resists sudden changes in ph. One mechanism the body uses to control blood ph involves the release of. describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g.,. the. What Are The Major Buffer In The Blood.
From www.slideserve.com
PPT Chapter 27 Fluid, Electrolyte and Acidbase Balance PowerPoint What Are The Major Buffer In The Blood a buffer is a solution that resists sudden changes in ph. This is responsible for about 80% of extracellular. The ph of the blood is maintained between 7.35 and 7.45 by an. One mechanism the body uses to control blood ph involves the release of. describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein. What Are The Major Buffer In The Blood.
From www.pinterest.com
Physiology Blood Buffer System Behrouz Human body facts What Are The Major Buffer In The Blood a buffer is a solution that resists sudden changes in ph. This is responsible for about 80% of extracellular. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in. the buffer systems. What Are The Major Buffer In The Blood.
From www.slideserve.com
PPT MLAB 2401 Clinical Chemistry Keri BrophyMartinez PowerPoint What Are The Major Buffer In The Blood describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g.,. One mechanism the body uses to control blood ph involves the release of. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood plasma include. What Are The Major Buffer In The Blood.
From www.slideserve.com
PPT MLAB 2401 Clinical Chemistry Keri BrophyMartinez PowerPoint What Are The Major Buffer In The Blood The ph of the blood is maintained between 7.35 and 7.45 by an. This is responsible for about 80% of extracellular. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in. describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their. What Are The Major Buffer In The Blood.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation What Are The Major Buffer In The Blood a buffer is a solution that resists sudden changes in ph. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g.,. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and. What Are The Major Buffer In The Blood.
From www.slideshare.net
Buffer in the blood What Are The Major Buffer In The Blood The ph of the blood is maintained between 7.35 and 7.45 by an. a buffer is a solution that resists sudden changes in ph. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and. What Are The Major Buffer In The Blood.
From www.slideserve.com
PPT Blood buffering system PowerPoint Presentation, free download What Are The Major Buffer In The Blood the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. One mechanism the body uses to control blood ph involves the release of. a buffer is a solution that resists sudden changes in ph. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The ph. What Are The Major Buffer In The Blood.
From www.youtube.com
Buffer action in the blood YouTube What Are The Major Buffer In The Blood the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. One mechanism the body uses to control blood ph involves the release of. This is responsible for about 80% of extracellular. describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g.,. The ph of the. What Are The Major Buffer In The Blood.
From www.slideserve.com
PPT Buffers and Salts PowerPoint Presentation, free download ID5405242 What Are The Major Buffer In The Blood the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. One mechanism the body uses to control blood ph involves the release of. The ph of the blood is maintained between 7.35 and 7.45 by an. describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system). What Are The Major Buffer In The Blood.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation What Are The Major Buffer In The Blood describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g.,. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in. a buffer is a solution that resists sudden changes in ph. This is responsible for about 80% of. What Are The Major Buffer In The Blood.
From en.ppt-online.org
Blood biochemistry online presentation What Are The Major Buffer In The Blood Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g.,. the buffer systems. What Are The Major Buffer In The Blood.
From www.slideserve.com
PPT Physiological system of blood. Functional importance of blood What Are The Major Buffer In The Blood describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g.,. a buffer is a solution that resists sudden changes in ph. The ph of the blood is maintained between 7.35 and 7.45 by an. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. What Are The Major Buffer In The Blood.
From www.slideshare.net
Blood buffer system What Are The Major Buffer In The Blood Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in. This is responsible for about 80% of extracellular. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic. What Are The Major Buffer In The Blood.
From www.slideserve.com
PPT Buffers and Titrations PowerPoint Presentation, free download What Are The Major Buffer In The Blood the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. The ph of the blood is maintained between 7.35 and 7.45 by an. One mechanism the body uses to control blood ph involves the release of. a buffer is a solution that resists sudden changes in ph. describe the major buffer systems of. What Are The Major Buffer In The Blood.
From beta.learner.org
The Buffer System in the Blood (animation) Annenberg Learner What Are The Major Buffer In The Blood One mechanism the body uses to control blood ph involves the release of. describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g.,. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. a buffer is a solution that resists sudden changes in ph.. What Are The Major Buffer In The Blood.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download What Are The Major Buffer In The Blood describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g.,. The ph of the blood is maintained between 7.35 and 7.45 by an. This is responsible for about 80% of extracellular. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. the buffer systems. What Are The Major Buffer In The Blood.
From www.slideserve.com
PPT Biological buffering of blood PowerPoint Presentation, free What Are The Major Buffer In The Blood This is responsible for about 80% of extracellular. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in. The ph of the blood is maintained between 7.35 and 7.45 by an. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. the buffer. What Are The Major Buffer In The Blood.