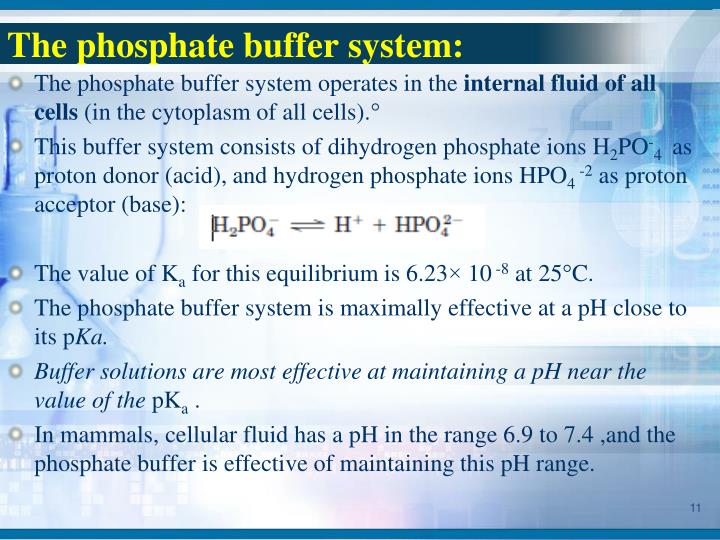
Biological Buffers Ph Range . The optimal buffering range for a buffer is the dissociation constant. Buffers should have a pka between 6.0 and 8.0 because the optimal ph for most biological reactions rests in this range. Almost all biological processes are ph dependent. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffers should have high water solubility and minimum. High water solubility and minimal organic solvent solubility. A first buffer (commonly, ph=7.0) is used to make major adjustments; A pka between 6.0 and 8.0. Then, a second buffer (ph=4.0) is used to make fine adjustments. Choose a buffer based on your ph requirements as well as the pka, a measure of acid strength that. We have developed a series of mixed biological buffers for protein analysis that can be used across a broad ph range, are compatible with. Choosing the right biological buffer. Even a slight change in ph can result in metabolic acidosis or alkalosis, resulting in severe. Buffers for biological systems should satisfy the following requirements:
from www.slideserve.com
The optimal buffering range for a buffer is the dissociation constant. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Choosing the right biological buffer. Buffers should have a pka between 6.0 and 8.0 because the optimal ph for most biological reactions rests in this range. Even a slight change in ph can result in metabolic acidosis or alkalosis, resulting in severe. Choose a buffer based on your ph requirements as well as the pka, a measure of acid strength that. Almost all biological processes are ph dependent. A first buffer (commonly, ph=7.0) is used to make major adjustments; We have developed a series of mixed biological buffers for protein analysis that can be used across a broad ph range, are compatible with. Then, a second buffer (ph=4.0) is used to make fine adjustments.
PPT Buffers of Biological & Clinical Significance PowerPoint
Biological Buffers Ph Range A pka between 6.0 and 8.0. Buffers should have a pka between 6.0 and 8.0 because the optimal ph for most biological reactions rests in this range. A pka between 6.0 and 8.0. The optimal buffering range for a buffer is the dissociation constant. A first buffer (commonly, ph=7.0) is used to make major adjustments; Then, a second buffer (ph=4.0) is used to make fine adjustments. Even a slight change in ph can result in metabolic acidosis or alkalosis, resulting in severe. Buffers for biological systems should satisfy the following requirements: We have developed a series of mixed biological buffers for protein analysis that can be used across a broad ph range, are compatible with. Buffers should have high water solubility and minimum. Choose a buffer based on your ph requirements as well as the pka, a measure of acid strength that. High water solubility and minimal organic solvent solubility. Almost all biological processes are ph dependent. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Choosing the right biological buffer.
From courses.lumenlearning.com
pH, Buffers, Acids, and Bases Introduction to Chemistry Biological Buffers Ph Range Choosing the right biological buffer. Buffers for biological systems should satisfy the following requirements: The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A pka between 6.0 and 8.0. High water solubility and minimal organic solvent solubility. A first buffer (commonly, ph=7.0) is used to make major adjustments; Buffers should have a. Biological Buffers Ph Range.
From www.scribd.com
pH and Buffers Acid Buffer Solution Biological Buffers Ph Range Buffers for biological systems should satisfy the following requirements: Buffers should have a pka between 6.0 and 8.0 because the optimal ph for most biological reactions rests in this range. The optimal buffering range for a buffer is the dissociation constant. A pka between 6.0 and 8.0. We have developed a series of mixed biological buffers for protein analysis that. Biological Buffers Ph Range.
From biotechnologymcq.com
Weak Acid Base PH Henderson Hasselbalch Equation Biological Buffer Biological Buffers Ph Range Choosing the right biological buffer. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Then, a second buffer (ph=4.0) is used to make fine adjustments. Buffers should have a pka between 6.0 and 8.0 because the optimal ph for most biological reactions rests in this range. Buffers should have high water solubility. Biological Buffers Ph Range.
From www.leinco.com
Biological Buffers And pH Range Leinco Technologies Biological Buffers Ph Range Choosing the right biological buffer. Buffers should have a pka between 6.0 and 8.0 because the optimal ph for most biological reactions rests in this range. We have developed a series of mixed biological buffers for protein analysis that can be used across a broad ph range, are compatible with. The optimal buffering range for a buffer is the dissociation. Biological Buffers Ph Range.
From iastate.pressbooks.pub
Properties of Blood as a Buffer and Blood Glucose A Mixed Course Biological Buffers Ph Range Buffers should have high water solubility and minimum. Buffers for biological systems should satisfy the following requirements: Buffers should have a pka between 6.0 and 8.0 because the optimal ph for most biological reactions rests in this range. A first buffer (commonly, ph=7.0) is used to make major adjustments; Then, a second buffer (ph=4.0) is used to make fine adjustments.. Biological Buffers Ph Range.
From solutionpharmacy.in
Applications Of Buffers Solution Parmacy Biological Buffers Ph Range We have developed a series of mixed biological buffers for protein analysis that can be used across a broad ph range, are compatible with. Then, a second buffer (ph=4.0) is used to make fine adjustments. Even a slight change in ph can result in metabolic acidosis or alkalosis, resulting in severe. Choosing the right biological buffer. Buffers should have a. Biological Buffers Ph Range.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Biological Buffers Ph Range Then, a second buffer (ph=4.0) is used to make fine adjustments. Buffers should have high water solubility and minimum. Choosing the right biological buffer. Choose a buffer based on your ph requirements as well as the pka, a measure of acid strength that. We have developed a series of mixed biological buffers for protein analysis that can be used across. Biological Buffers Ph Range.
From www.crawfordscientific.com
Buffer choice for HPLC separations Biological Buffers Ph Range Choose a buffer based on your ph requirements as well as the pka, a measure of acid strength that. Buffers should have high water solubility and minimum. Even a slight change in ph can result in metabolic acidosis or alkalosis, resulting in severe. A first buffer (commonly, ph=7.0) is used to make major adjustments; The optimal buffering range for a. Biological Buffers Ph Range.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Biological Buffers Ph Range A first buffer (commonly, ph=7.0) is used to make major adjustments; Buffers should have high water solubility and minimum. High water solubility and minimal organic solvent solubility. Choose a buffer based on your ph requirements as well as the pka, a measure of acid strength that. Buffers for biological systems should satisfy the following requirements: The optimal buffering range for. Biological Buffers Ph Range.
From www.youtube.com
Calculating the pH of a buffer YouTube Biological Buffers Ph Range Then, a second buffer (ph=4.0) is used to make fine adjustments. High water solubility and minimal organic solvent solubility. Choosing the right biological buffer. Choose a buffer based on your ph requirements as well as the pka, a measure of acid strength that. We have developed a series of mixed biological buffers for protein analysis that can be used across. Biological Buffers Ph Range.
From www.zirchrom.com
Useful HPLC Information Biological Buffers Ph Range The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The optimal buffering range for a buffer is the dissociation constant. Even a slight change in ph can result in metabolic acidosis or alkalosis, resulting in severe. Buffers for biological systems should satisfy the following requirements: We have developed a series of mixed. Biological Buffers Ph Range.
From bitesizebio.com
How Do Buffers Work? An Easy Explaination for Biologists Biological Buffers Ph Range Choosing the right biological buffer. Even a slight change in ph can result in metabolic acidosis or alkalosis, resulting in severe. Almost all biological processes are ph dependent. High water solubility and minimal organic solvent solubility. A first buffer (commonly, ph=7.0) is used to make major adjustments; Then, a second buffer (ph=4.0) is used to make fine adjustments. Buffers should. Biological Buffers Ph Range.
From www.hopaxfc.com
Biological Buffers Products Hopax Fine Chemicals Biological Buffers Ph Range We have developed a series of mixed biological buffers for protein analysis that can be used across a broad ph range, are compatible with. Then, a second buffer (ph=4.0) is used to make fine adjustments. Almost all biological processes are ph dependent. Buffers should have high water solubility and minimum. Buffers should have a pka between 6.0 and 8.0 because. Biological Buffers Ph Range.
From www.semanticscholar.org
[PDF] HPLC Columns John Dolan A Guide to HPLC and LCMS Buffer Biological Buffers Ph Range The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Even a slight change in ph can result in metabolic acidosis or alkalosis, resulting in severe. Choose a buffer based on your ph requirements as well as the pka, a measure of acid strength that. Buffers for biological systems should satisfy the following. Biological Buffers Ph Range.
From www.researchgate.net
Any suggestion of buffer solution for photocatalysis using ZnO Biological Buffers Ph Range A first buffer (commonly, ph=7.0) is used to make major adjustments; Buffers should have a pka between 6.0 and 8.0 because the optimal ph for most biological reactions rests in this range. We have developed a series of mixed biological buffers for protein analysis that can be used across a broad ph range, are compatible with. Even a slight change. Biological Buffers Ph Range.
From www.youtube.com
Buffers and pH Biology YouTube Biological Buffers Ph Range The optimal buffering range for a buffer is the dissociation constant. We have developed a series of mixed biological buffers for protein analysis that can be used across a broad ph range, are compatible with. A pka between 6.0 and 8.0. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Choose a. Biological Buffers Ph Range.
From labpedia.net
Acidbase Balance Part 2 Introduction of AcidBase Balance Biological Buffers Ph Range Choose a buffer based on your ph requirements as well as the pka, a measure of acid strength that. We have developed a series of mixed biological buffers for protein analysis that can be used across a broad ph range, are compatible with. A first buffer (commonly, ph=7.0) is used to make major adjustments; Almost all biological processes are ph. Biological Buffers Ph Range.
From www.researchgate.net
1. Buffer solutions preparation method Download Table Biological Buffers Ph Range Buffers for biological systems should satisfy the following requirements: High water solubility and minimal organic solvent solubility. Buffers should have a pka between 6.0 and 8.0 because the optimal ph for most biological reactions rests in this range. Even a slight change in ph can result in metabolic acidosis or alkalosis, resulting in severe. A first buffer (commonly, ph=7.0) is. Biological Buffers Ph Range.
From www.slideshare.net
02 hydrolysis. buffers__colloids Biological Buffers Ph Range Even a slight change in ph can result in metabolic acidosis or alkalosis, resulting in severe. Buffers should have high water solubility and minimum. The optimal buffering range for a buffer is the dissociation constant. Choosing the right biological buffer. A first buffer (commonly, ph=7.0) is used to make major adjustments; The buffer systems functioning in blood plasma include plasma. Biological Buffers Ph Range.
From pdfprof.com
buffer capacity experiment procedure Biological Buffers Ph Range Then, a second buffer (ph=4.0) is used to make fine adjustments. Buffers should have a pka between 6.0 and 8.0 because the optimal ph for most biological reactions rests in this range. A first buffer (commonly, ph=7.0) is used to make major adjustments; Buffers should have high water solubility and minimum. Choosing the right biological buffer. A pka between 6.0. Biological Buffers Ph Range.
From medium.com
The impact of temperature changes on biological buffers’ pKa by Biological Buffers Ph Range We have developed a series of mixed biological buffers for protein analysis that can be used across a broad ph range, are compatible with. Then, a second buffer (ph=4.0) is used to make fine adjustments. High water solubility and minimal organic solvent solubility. The optimal buffering range for a buffer is the dissociation constant. The buffer systems functioning in blood. Biological Buffers Ph Range.
From saylordotorg.github.io
Buffers Biological Buffers Ph Range The optimal buffering range for a buffer is the dissociation constant. Almost all biological processes are ph dependent. A pka between 6.0 and 8.0. We have developed a series of mixed biological buffers for protein analysis that can be used across a broad ph range, are compatible with. Buffers for biological systems should satisfy the following requirements: Then, a second. Biological Buffers Ph Range.
From www.researchgate.net
Examples of buffer compounds divided into accepted physiological Biological Buffers Ph Range High water solubility and minimal organic solvent solubility. Choosing the right biological buffer. Buffers should have a pka between 6.0 and 8.0 because the optimal ph for most biological reactions rests in this range. A first buffer (commonly, ph=7.0) is used to make major adjustments; A pka between 6.0 and 8.0. Then, a second buffer (ph=4.0) is used to make. Biological Buffers Ph Range.
From www.researchgate.net
The effect of buffer species and environmental pH on immunoglobulin G Biological Buffers Ph Range Then, a second buffer (ph=4.0) is used to make fine adjustments. Choosing the right biological buffer. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffers should have a pka between 6.0 and 8.0 because the optimal ph for most biological reactions rests in this range. High water solubility and minimal organic. Biological Buffers Ph Range.
From www.myxxgirl.com
Buffer System Classification Example And Mechanism Of Action My XXX Biological Buffers Ph Range Then, a second buffer (ph=4.0) is used to make fine adjustments. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffers should have a pka between 6.0 and 8.0 because the optimal ph for most biological reactions rests in this range. The optimal buffering range for a buffer is the dissociation constant.. Biological Buffers Ph Range.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Biological Buffers Ph Range High water solubility and minimal organic solvent solubility. Buffers should have high water solubility and minimum. A first buffer (commonly, ph=7.0) is used to make major adjustments; Choosing the right biological buffer. Almost all biological processes are ph dependent. Even a slight change in ph can result in metabolic acidosis or alkalosis, resulting in severe. A pka between 6.0 and. Biological Buffers Ph Range.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation, free download ID496487 Biological Buffers Ph Range A pka between 6.0 and 8.0. A first buffer (commonly, ph=7.0) is used to make major adjustments; Buffers should have a pka between 6.0 and 8.0 because the optimal ph for most biological reactions rests in this range. Choose a buffer based on your ph requirements as well as the pka, a measure of acid strength that. Buffers for biological. Biological Buffers Ph Range.
From www.fishersci.com
Quality Biological Inc HEPES Buffer (1 M), pH 7.3, PK 4 X 100 mL Biological Buffers Ph Range The optimal buffering range for a buffer is the dissociation constant. A first buffer (commonly, ph=7.0) is used to make major adjustments; Then, a second buffer (ph=4.0) is used to make fine adjustments. Buffers should have high water solubility and minimum. Buffers for biological systems should satisfy the following requirements: Even a slight change in ph can result in metabolic. Biological Buffers Ph Range.
From www.researchgate.net
(PDF) Biological pH buffers in IVF Help or hindrance to success Biological Buffers Ph Range Almost all biological processes are ph dependent. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffers for biological systems should satisfy the following requirements: A pka between 6.0 and 8.0. Then, a second buffer (ph=4.0) is used to make fine adjustments. We have developed a series of mixed biological buffers for. Biological Buffers Ph Range.
From www.slideshare.net
Ch 18 buffers Biological Buffers Ph Range Choosing the right biological buffer. A first buffer (commonly, ph=7.0) is used to make major adjustments; We have developed a series of mixed biological buffers for protein analysis that can be used across a broad ph range, are compatible with. Buffers should have a pka between 6.0 and 8.0 because the optimal ph for most biological reactions rests in this. Biological Buffers Ph Range.
From www.semanticscholar.org
[PDF] (Un)suitability of the use of pH buffers in biological Biological Buffers Ph Range Choose a buffer based on your ph requirements as well as the pka, a measure of acid strength that. Buffers should have a pka between 6.0 and 8.0 because the optimal ph for most biological reactions rests in this range. Almost all biological processes are ph dependent. Buffers for biological systems should satisfy the following requirements: The buffer systems functioning. Biological Buffers Ph Range.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Biological Buffers Ph Range High water solubility and minimal organic solvent solubility. Buffers should have a pka between 6.0 and 8.0 because the optimal ph for most biological reactions rests in this range. A first buffer (commonly, ph=7.0) is used to make major adjustments; We have developed a series of mixed biological buffers for protein analysis that can be used across a broad ph. Biological Buffers Ph Range.
From studylib.net
Good's buffers (biological buffers) Biological Buffers Ph Range Choosing the right biological buffer. Buffers for biological systems should satisfy the following requirements: Then, a second buffer (ph=4.0) is used to make fine adjustments. The optimal buffering range for a buffer is the dissociation constant. High water solubility and minimal organic solvent solubility. We have developed a series of mixed biological buffers for protein analysis that can be used. Biological Buffers Ph Range.
From dandkmotorsports.com
Hepes Buffer Recipe Ph 7 2 Dandk Organizer Biological Buffers Ph Range Choosing the right biological buffer. Then, a second buffer (ph=4.0) is used to make fine adjustments. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Almost all biological processes are ph dependent. A first buffer (commonly, ph=7.0) is used to make major adjustments; Choose a buffer based on your ph requirements as. Biological Buffers Ph Range.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Biological Buffers Ph Range Choose a buffer based on your ph requirements as well as the pka, a measure of acid strength that. High water solubility and minimal organic solvent solubility. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffers should have high water solubility and minimum. We have developed a series of mixed biological. Biological Buffers Ph Range.