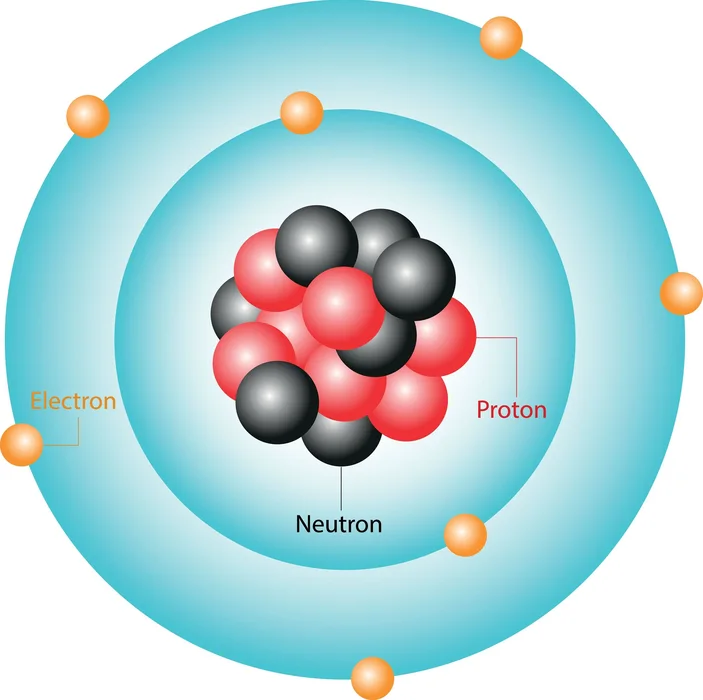
Bohr Atomic Model Limitations . The bohr model of the atom, proposed by niels bohr in 1913, describes electrons as orbiting the nucleus in circular orbits with quantized energy levels. It does not comply with the. Compare bohr's model with rutherford's model and see examples of bohr models for carbon, oxygen and sodium atoms. Learn how bohr's model explained atomic emission spectra, energy levels, and electron stability, and its limitations for complex atoms. Limitations of bohr’s atomic model. The following are the basic limitations of bohr’s model of the hydrogen atom. Limitations of bohr's atomic model. Bohr's model combines principles from both classical and quantum physics. Learn about the postulates, merits and limitations of bohr's atomic model, which explains the structure and spectra of hydrogen and other atoms. Bohr's model of the atom has several limitations. Find out how it evolved from earlier models and what are its. Find out the equations for radius, energy, and angular momentum of the electron. Learn about the bohr model of the atom, a quantum theory that explains the spectral lines of hydrogen and other elements.
from 88guru.com
Learn how bohr's model explained atomic emission spectra, energy levels, and electron stability, and its limitations for complex atoms. Bohr's model combines principles from both classical and quantum physics. Limitations of bohr's atomic model. It does not comply with the. The following are the basic limitations of bohr’s model of the hydrogen atom. Learn about the bohr model of the atom, a quantum theory that explains the spectral lines of hydrogen and other elements. Find out the equations for radius, energy, and angular momentum of the electron. The bohr model of the atom, proposed by niels bohr in 1913, describes electrons as orbiting the nucleus in circular orbits with quantized energy levels. Limitations of bohr’s atomic model. Bohr's model of the atom has several limitations.
Bohr's Atomic Model Theory and Its Limitations
Bohr Atomic Model Limitations Learn about the bohr model of the atom, a quantum theory that explains the spectral lines of hydrogen and other elements. Learn about the postulates, merits and limitations of bohr's atomic model, which explains the structure and spectra of hydrogen and other atoms. Limitations of bohr's atomic model. Bohr's model combines principles from both classical and quantum physics. Learn about the bohr model of the atom, a quantum theory that explains the spectral lines of hydrogen and other elements. Compare bohr's model with rutherford's model and see examples of bohr models for carbon, oxygen and sodium atoms. The following are the basic limitations of bohr’s model of the hydrogen atom. The bohr model of the atom, proposed by niels bohr in 1913, describes electrons as orbiting the nucleus in circular orbits with quantized energy levels. Limitations of bohr’s atomic model. Find out the equations for radius, energy, and angular momentum of the electron. It does not comply with the. Bohr's model of the atom has several limitations. Learn how bohr's model explained atomic emission spectra, energy levels, and electron stability, and its limitations for complex atoms. Find out how it evolved from earlier models and what are its.
From www.chemistrylearner.com
Bohr Model Definition, Features, and Limitations Bohr Atomic Model Limitations Find out how it evolved from earlier models and what are its. Compare bohr's model with rutherford's model and see examples of bohr models for carbon, oxygen and sodium atoms. Learn how bohr's model explained atomic emission spectra, energy levels, and electron stability, and its limitations for complex atoms. Bohr's model combines principles from both classical and quantum physics. It. Bohr Atomic Model Limitations.
From www.youtube.com
Bohr Atomic Model Features and Limitations YouTube Bohr Atomic Model Limitations It does not comply with the. The bohr model of the atom, proposed by niels bohr in 1913, describes electrons as orbiting the nucleus in circular orbits with quantized energy levels. Limitations of bohr's atomic model. The following are the basic limitations of bohr’s model of the hydrogen atom. Compare bohr's model with rutherford's model and see examples of bohr. Bohr Atomic Model Limitations.
From www.youtube.com
Defects of Bohr's Atomic Model Bohrs Atomic Theory Drawbacks Bohr Atomic Model Limitations Limitations of bohr’s atomic model. The following are the basic limitations of bohr’s model of the hydrogen atom. Bohr's model combines principles from both classical and quantum physics. It does not comply with the. Learn about the postulates, merits and limitations of bohr's atomic model, which explains the structure and spectra of hydrogen and other atoms. Bohr's model of the. Bohr Atomic Model Limitations.
From 88guru.com
Bohr's Atomic Model Theory and Its Limitations Bohr Atomic Model Limitations Find out how it evolved from earlier models and what are its. Bohr's model of the atom has several limitations. Find out the equations for radius, energy, and angular momentum of the electron. The bohr model of the atom, proposed by niels bohr in 1913, describes electrons as orbiting the nucleus in circular orbits with quantized energy levels. Limitations of. Bohr Atomic Model Limitations.
From www.youtube.com
7. 11C02.4 CV 4 Limitation of Bohr's Model YouTube Bohr Atomic Model Limitations Find out the equations for radius, energy, and angular momentum of the electron. Find out how it evolved from earlier models and what are its. Learn about the bohr model of the atom, a quantum theory that explains the spectral lines of hydrogen and other elements. Limitations of bohr’s atomic model. Limitations of bohr's atomic model. It does not comply. Bohr Atomic Model Limitations.
From slideplayer.com
The Spectrum and the Model of the Atom Part I ppt Bohr Atomic Model Limitations Bohr's model combines principles from both classical and quantum physics. The following are the basic limitations of bohr’s model of the hydrogen atom. Compare bohr's model with rutherford's model and see examples of bohr models for carbon, oxygen and sodium atoms. Find out how it evolved from earlier models and what are its. Learn how bohr's model explained atomic emission. Bohr Atomic Model Limitations.
From www.thoughtco.com
Bohr Model of the Atom Overview and Examples Bohr Atomic Model Limitations Compare bohr's model with rutherford's model and see examples of bohr models for carbon, oxygen and sodium atoms. It does not comply with the. Bohr's model of the atom has several limitations. Learn about the postulates, merits and limitations of bohr's atomic model, which explains the structure and spectra of hydrogen and other atoms. The following are the basic limitations. Bohr Atomic Model Limitations.
From www.studypool.com
SOLUTION Limitations of bohr s atomic model Studypool Bohr Atomic Model Limitations Bohr's model combines principles from both classical and quantum physics. The bohr model of the atom, proposed by niels bohr in 1913, describes electrons as orbiting the nucleus in circular orbits with quantized energy levels. Bohr's model of the atom has several limitations. Learn how bohr's model explained atomic emission spectra, energy levels, and electron stability, and its limitations for. Bohr Atomic Model Limitations.
From scienceready.com.au
Bohr's Atomic Model & Limitations HSC Physics Science Ready Bohr Atomic Model Limitations Learn about the bohr model of the atom, a quantum theory that explains the spectral lines of hydrogen and other elements. Find out how it evolved from earlier models and what are its. It does not comply with the. Limitations of bohr’s atomic model. Bohr's model combines principles from both classical and quantum physics. Learn about the postulates, merits and. Bohr Atomic Model Limitations.
From www.teachoo.com
Bohr's Model of Atom Postulate and it's limitations Teachoo Bohr Atomic Model Limitations Bohr's model of the atom has several limitations. Find out the equations for radius, energy, and angular momentum of the electron. The following are the basic limitations of bohr’s model of the hydrogen atom. It does not comply with the. Limitations of bohr’s atomic model. Find out how it evolved from earlier models and what are its. Limitations of bohr's. Bohr Atomic Model Limitations.
From www.youtube.com
Bohr Atomic Model, Rydberg's Equation, Hydrogen Spectrum // HSC Physics Bohr Atomic Model Limitations Bohr's model of the atom has several limitations. Learn about the postulates, merits and limitations of bohr's atomic model, which explains the structure and spectra of hydrogen and other atoms. Learn about the bohr model of the atom, a quantum theory that explains the spectral lines of hydrogen and other elements. It does not comply with the. Find out the. Bohr Atomic Model Limitations.
From gbu-taganskij.ru
Bohr's Atomic Model Postulates Diagram Limitations, 47 OFF Bohr Atomic Model Limitations Bohr's model combines principles from both classical and quantum physics. Compare bohr's model with rutherford's model and see examples of bohr models for carbon, oxygen and sodium atoms. Learn how bohr's model explained atomic emission spectra, energy levels, and electron stability, and its limitations for complex atoms. Limitations of bohr's atomic model. Find out how it evolved from earlier models. Bohr Atomic Model Limitations.
From pdfprof.com
modèle planétaire de bohr pdf Bohr Atomic Model Limitations Find out how it evolved from earlier models and what are its. Find out the equations for radius, energy, and angular momentum of the electron. The following are the basic limitations of bohr’s model of the hydrogen atom. Learn about the postulates, merits and limitations of bohr's atomic model, which explains the structure and spectra of hydrogen and other atoms.. Bohr Atomic Model Limitations.
From proper-cooking.info
Bohrs Model Project Bohr Atomic Model Limitations Limitations of bohr’s atomic model. Bohr's model combines principles from both classical and quantum physics. Compare bohr's model with rutherford's model and see examples of bohr models for carbon, oxygen and sodium atoms. The bohr model of the atom, proposed by niels bohr in 1913, describes electrons as orbiting the nucleus in circular orbits with quantized energy levels. Bohr's model. Bohr Atomic Model Limitations.
From chemistrylearninghub.com
BOHR ATOMIC MODEL / INTRODUCTION/POSTULATES/DEFECTS CHEMISTRY Bohr Atomic Model Limitations Bohr's model of the atom has several limitations. The following are the basic limitations of bohr’s model of the hydrogen atom. Compare bohr's model with rutherford's model and see examples of bohr models for carbon, oxygen and sodium atoms. Learn how bohr's model explained atomic emission spectra, energy levels, and electron stability, and its limitations for complex atoms. Limitations of. Bohr Atomic Model Limitations.
From www.youtube.com
BOHR'S ATOMIC MODEL THE POSTULATES YouTube Bohr Atomic Model Limitations Limitations of bohr’s atomic model. The bohr model of the atom, proposed by niels bohr in 1913, describes electrons as orbiting the nucleus in circular orbits with quantized energy levels. Learn about the bohr model of the atom, a quantum theory that explains the spectral lines of hydrogen and other elements. The following are the basic limitations of bohr’s model. Bohr Atomic Model Limitations.
From brunofuga.adv.br
Bohr's Model Of An Atom Limitations, Examples, And FAQs, 57 OFF Bohr Atomic Model Limitations Compare bohr's model with rutherford's model and see examples of bohr models for carbon, oxygen and sodium atoms. Find out the equations for radius, energy, and angular momentum of the electron. The bohr model of the atom, proposed by niels bohr in 1913, describes electrons as orbiting the nucleus in circular orbits with quantized energy levels. Limitations of bohr’s atomic. Bohr Atomic Model Limitations.
From classnotes.org.in
Bohr's Model of an Atom Chemistry, Class 11, Structure of Atom Bohr Atomic Model Limitations It does not comply with the. The following are the basic limitations of bohr’s model of the hydrogen atom. Bohr's model of the atom has several limitations. Learn about the postulates, merits and limitations of bohr's atomic model, which explains the structure and spectra of hydrogen and other atoms. Limitations of bohr's atomic model. Compare bohr's model with rutherford's model. Bohr Atomic Model Limitations.
From www.scribd.com
Bohrs Atomic Model and Its Limitations PDF Bohr Atomic Model Limitations The bohr model of the atom, proposed by niels bohr in 1913, describes electrons as orbiting the nucleus in circular orbits with quantized energy levels. Find out the equations for radius, energy, and angular momentum of the electron. Bohr's model combines principles from both classical and quantum physics. Learn about the bohr model of the atom, a quantum theory that. Bohr Atomic Model Limitations.
From www.teachoo.com
Drawbacks of Rutherford's Atomic Model Experiment [with Examples] Bohr Atomic Model Limitations Find out the equations for radius, energy, and angular momentum of the electron. Learn how bohr's model explained atomic emission spectra, energy levels, and electron stability, and its limitations for complex atoms. Bohr's model combines principles from both classical and quantum physics. It does not comply with the. Limitations of bohr's atomic model. Learn about the postulates, merits and limitations. Bohr Atomic Model Limitations.
From scienceready.com.au
M8S7 Bohr's and Rutherford's Atomic Models and their Limitations Bohr Atomic Model Limitations Bohr's model combines principles from both classical and quantum physics. It does not comply with the. Bohr's model of the atom has several limitations. The following are the basic limitations of bohr’s model of the hydrogen atom. Limitations of bohr’s atomic model. Find out the equations for radius, energy, and angular momentum of the electron. The bohr model of the. Bohr Atomic Model Limitations.
From www.youtube.com
Bohrs Atomic Model Postulates Bohr Model of Atom Chemistry Chapter Bohr Atomic Model Limitations It does not comply with the. Limitations of bohr’s atomic model. Find out the equations for radius, energy, and angular momentum of the electron. Bohr's model of the atom has several limitations. The bohr model of the atom, proposed by niels bohr in 1913, describes electrons as orbiting the nucleus in circular orbits with quantized energy levels. Compare bohr's model. Bohr Atomic Model Limitations.
From www.teachoo.com
Bohr's Model of Atom Postulate and it's limitations Teachoo Bohr Atomic Model Limitations Learn about the bohr model of the atom, a quantum theory that explains the spectral lines of hydrogen and other elements. Learn how bohr's model explained atomic emission spectra, energy levels, and electron stability, and its limitations for complex atoms. Bohr's model combines principles from both classical and quantum physics. Bohr's model of the atom has several limitations. Limitations of. Bohr Atomic Model Limitations.
From byjus.com
Write the postulates of Bohr's atom model. Bohr Atomic Model Limitations Limitations of bohr's atomic model. The following are the basic limitations of bohr’s model of the hydrogen atom. Find out how it evolved from earlier models and what are its. Bohr's model combines principles from both classical and quantum physics. The bohr model of the atom, proposed by niels bohr in 1913, describes electrons as orbiting the nucleus in circular. Bohr Atomic Model Limitations.
From www.expii.com
Bohr's Atomic Model — Overview & Importance Expii Bohr Atomic Model Limitations Bohr's model combines principles from both classical and quantum physics. Find out the equations for radius, energy, and angular momentum of the electron. It does not comply with the. Limitations of bohr’s atomic model. Learn how bohr's model explained atomic emission spectra, energy levels, and electron stability, and its limitations for complex atoms. Limitations of bohr's atomic model. Compare bohr's. Bohr Atomic Model Limitations.
From printablefugintko.z4.web.core.windows.net
Describe The Bohr Model Of The Atom Bohr Atomic Model Limitations Learn about the bohr model of the atom, a quantum theory that explains the spectral lines of hydrogen and other elements. Bohr's model combines principles from both classical and quantum physics. The bohr model of the atom, proposed by niels bohr in 1913, describes electrons as orbiting the nucleus in circular orbits with quantized energy levels. The following are the. Bohr Atomic Model Limitations.
From 88guru.com
Bohr's Atomic Model Theory and Its Limitations Bohr Atomic Model Limitations Learn about the postulates, merits and limitations of bohr's atomic model, which explains the structure and spectra of hydrogen and other atoms. Limitations of bohr’s atomic model. Bohr's model of the atom has several limitations. Learn how bohr's model explained atomic emission spectra, energy levels, and electron stability, and its limitations for complex atoms. The bohr model of the atom,. Bohr Atomic Model Limitations.
From scienceready.com.au
Bohr's Atomic Model & Limitations HSC Physics Science Ready Bohr Atomic Model Limitations The following are the basic limitations of bohr’s model of the hydrogen atom. Learn about the bohr model of the atom, a quantum theory that explains the spectral lines of hydrogen and other elements. It does not comply with the. Find out the equations for radius, energy, and angular momentum of the electron. Bohr's model combines principles from both classical. Bohr Atomic Model Limitations.
From chemistnotes.com
Bohr's Atomic Model, Postulates, Merits and Limitations Chemistry Notes Bohr Atomic Model Limitations Find out the equations for radius, energy, and angular momentum of the electron. Limitations of bohr’s atomic model. The bohr model of the atom, proposed by niels bohr in 1913, describes electrons as orbiting the nucleus in circular orbits with quantized energy levels. Bohr's model of the atom has several limitations. Limitations of bohr's atomic model. It does not comply. Bohr Atomic Model Limitations.
From klaasxqyg.blob.core.windows.net
How To Use A Bohr Model at Veronica Hill blog Bohr Atomic Model Limitations Learn how bohr's model explained atomic emission spectra, energy levels, and electron stability, and its limitations for complex atoms. Learn about the bohr model of the atom, a quantum theory that explains the spectral lines of hydrogen and other elements. Bohr's model combines principles from both classical and quantum physics. Compare bohr's model with rutherford's model and see examples of. Bohr Atomic Model Limitations.
From ar.inspiredpencil.com
Bohrs Atomic Model Labeled Bohr Atomic Model Limitations Bohr's model combines principles from both classical and quantum physics. Bohr's model of the atom has several limitations. Find out how it evolved from earlier models and what are its. Find out the equations for radius, energy, and angular momentum of the electron. Learn about the bohr model of the atom, a quantum theory that explains the spectral lines of. Bohr Atomic Model Limitations.
From worksheetcampusmantra.z13.web.core.windows.net
Bohr Atomic Model Explained Bohr Atomic Model Limitations Find out the equations for radius, energy, and angular momentum of the electron. Bohr's model combines principles from both classical and quantum physics. Learn about the postulates, merits and limitations of bohr's atomic model, which explains the structure and spectra of hydrogen and other atoms. Compare bohr's model with rutherford's model and see examples of bohr models for carbon, oxygen. Bohr Atomic Model Limitations.
From images.modishstore.com
Modelo Atomico De Niels Bohr MODISEDU Bohr Atomic Model Limitations The following are the basic limitations of bohr’s model of the hydrogen atom. Limitations of bohr's atomic model. Bohr's model of the atom has several limitations. The bohr model of the atom, proposed by niels bohr in 1913, describes electrons as orbiting the nucleus in circular orbits with quantized energy levels. Bohr's model combines principles from both classical and quantum. Bohr Atomic Model Limitations.
From slideplayer.com
The Bohr Model of the Atom ppt download Bohr Atomic Model Limitations Find out how it evolved from earlier models and what are its. Bohr's model combines principles from both classical and quantum physics. Limitations of bohr’s atomic model. Learn how bohr's model explained atomic emission spectra, energy levels, and electron stability, and its limitations for complex atoms. It does not comply with the. Learn about the bohr model of the atom,. Bohr Atomic Model Limitations.
From www.youtube.com
Limitations of Bohrs's Atomic ModelNuclear Physics5thSEMB.SC+3 Bohr Atomic Model Limitations It does not comply with the. Limitations of bohr’s atomic model. The bohr model of the atom, proposed by niels bohr in 1913, describes electrons as orbiting the nucleus in circular orbits with quantized energy levels. Find out how it evolved from earlier models and what are its. Learn about the bohr model of the atom, a quantum theory that. Bohr Atomic Model Limitations.