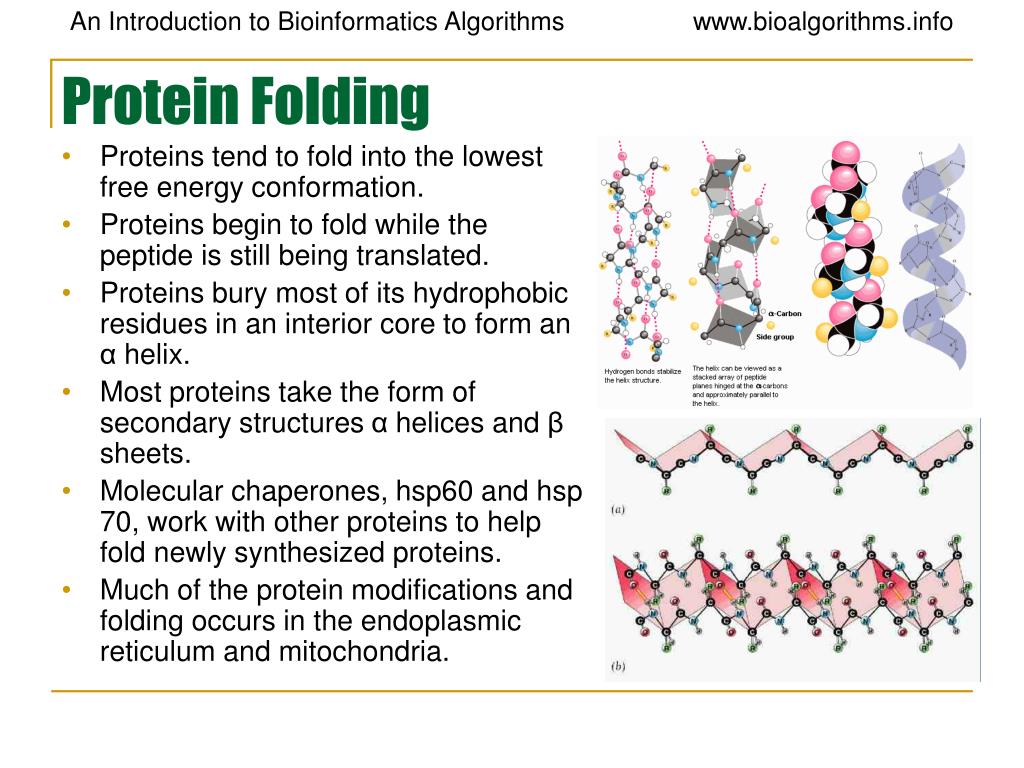
Protein Folding Is Primarily Driven By . this chapter discusses the vast conformational space accessible to a protein chain, the diversity of weak noncovalent. the cornerstone assumption in the field of protein folding is that proteins spontaneously fold into their native. function in proteins largely depends on the acquisition of specific. back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field; in vitro experiments involve denaturing the protein with urea, guanidine hydrochloride, or heat, then refolding the protein by. we have learned that proteins fold rapidly because random thermal motions cause conformational changes leading.
from www.slideserve.com
the cornerstone assumption in the field of protein folding is that proteins spontaneously fold into their native. this chapter discusses the vast conformational space accessible to a protein chain, the diversity of weak noncovalent. we have learned that proteins fold rapidly because random thermal motions cause conformational changes leading. function in proteins largely depends on the acquisition of specific. in vitro experiments involve denaturing the protein with urea, guanidine hydrochloride, or heat, then refolding the protein by. back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field;
PPT Molecular Biology Primer PowerPoint Presentation, free download ID1436519
Protein Folding Is Primarily Driven By in vitro experiments involve denaturing the protein with urea, guanidine hydrochloride, or heat, then refolding the protein by. function in proteins largely depends on the acquisition of specific. we have learned that proteins fold rapidly because random thermal motions cause conformational changes leading. this chapter discusses the vast conformational space accessible to a protein chain, the diversity of weak noncovalent. back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field; the cornerstone assumption in the field of protein folding is that proteins spontaneously fold into their native. in vitro experiments involve denaturing the protein with urea, guanidine hydrochloride, or heat, then refolding the protein by.
From www.pnas.org
An amino acid code for protein folding PNAS Protein Folding Is Primarily Driven By the cornerstone assumption in the field of protein folding is that proteins spontaneously fold into their native. back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field; function in proteins largely depends on the acquisition of specific. in vitro experiments involve denaturing the protein with urea,. Protein Folding Is Primarily Driven By.
From www.pnas.org
The case for defined protein folding pathways PNAS Protein Folding Is Primarily Driven By we have learned that proteins fold rapidly because random thermal motions cause conformational changes leading. this chapter discusses the vast conformational space accessible to a protein chain, the diversity of weak noncovalent. the cornerstone assumption in the field of protein folding is that proteins spontaneously fold into their native. back in the 1980s and 1990s, it. Protein Folding Is Primarily Driven By.
From www.slideserve.com
PPT Protein Folding Prediction PowerPoint Presentation, free download ID4819731 Protein Folding Is Primarily Driven By we have learned that proteins fold rapidly because random thermal motions cause conformational changes leading. this chapter discusses the vast conformational space accessible to a protein chain, the diversity of weak noncovalent. the cornerstone assumption in the field of protein folding is that proteins spontaneously fold into their native. back in the 1980s and 1990s, it. Protein Folding Is Primarily Driven By.
From dxobzlucv.blob.core.windows.net
Protein Folding Occurs After The Process Of at Nichole Cobb blog Protein Folding Is Primarily Driven By in vitro experiments involve denaturing the protein with urea, guanidine hydrochloride, or heat, then refolding the protein by. function in proteins largely depends on the acquisition of specific. this chapter discusses the vast conformational space accessible to a protein chain, the diversity of weak noncovalent. the cornerstone assumption in the field of protein folding is that. Protein Folding Is Primarily Driven By.
From slidetodoc.com
Protein Folding Processing and Degradation Protein Folding Protein Protein Folding Is Primarily Driven By this chapter discusses the vast conformational space accessible to a protein chain, the diversity of weak noncovalent. we have learned that proteins fold rapidly because random thermal motions cause conformational changes leading. back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field; function in proteins largely. Protein Folding Is Primarily Driven By.
From www.slideserve.com
PPT Protein Synthesis and Protein Folding PowerPoint Presentation, free download ID2804584 Protein Folding Is Primarily Driven By this chapter discusses the vast conformational space accessible to a protein chain, the diversity of weak noncovalent. in vitro experiments involve denaturing the protein with urea, guanidine hydrochloride, or heat, then refolding the protein by. the cornerstone assumption in the field of protein folding is that proteins spontaneously fold into their native. function in proteins largely. Protein Folding Is Primarily Driven By.
From www.researchgate.net
A folding fitness peak describing relationships of protein folding,... Download Scientific Diagram Protein Folding Is Primarily Driven By in vitro experiments involve denaturing the protein with urea, guanidine hydrochloride, or heat, then refolding the protein by. the cornerstone assumption in the field of protein folding is that proteins spontaneously fold into their native. this chapter discusses the vast conformational space accessible to a protein chain, the diversity of weak noncovalent. back in the 1980s. Protein Folding Is Primarily Driven By.
From www.slideserve.com
PPT Proteomics PowerPoint Presentation, free download ID430949 Protein Folding Is Primarily Driven By in vitro experiments involve denaturing the protein with urea, guanidine hydrochloride, or heat, then refolding the protein by. the cornerstone assumption in the field of protein folding is that proteins spontaneously fold into their native. function in proteins largely depends on the acquisition of specific. back in the 1980s and 1990s, it was the desire to. Protein Folding Is Primarily Driven By.
From www.youtube.com
Protein Folding Basics, Driving Forces, Experimental Techniques, Challenges & Applications Protein Folding Is Primarily Driven By back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field; the cornerstone assumption in the field of protein folding is that proteins spontaneously fold into their native. we have learned that proteins fold rapidly because random thermal motions cause conformational changes leading. function in proteins largely. Protein Folding Is Primarily Driven By.
From phys.org
Folding proteins feel the heat, and cold Protein Folding Is Primarily Driven By in vitro experiments involve denaturing the protein with urea, guanidine hydrochloride, or heat, then refolding the protein by. function in proteins largely depends on the acquisition of specific. the cornerstone assumption in the field of protein folding is that proteins spontaneously fold into their native. back in the 1980s and 1990s, it was the desire to. Protein Folding Is Primarily Driven By.
From en.wikipedia.org
Protein biosynthesis Wikipedia Protein Folding Is Primarily Driven By back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field; the cornerstone assumption in the field of protein folding is that proteins spontaneously fold into their native. this chapter discusses the vast conformational space accessible to a protein chain, the diversity of weak noncovalent. we have. Protein Folding Is Primarily Driven By.
From www.winemakersresearchexchange.com
Winemaker's Research Exchange Protein folding explains protein instability in wine Protein Folding Is Primarily Driven By this chapter discusses the vast conformational space accessible to a protein chain, the diversity of weak noncovalent. in vitro experiments involve denaturing the protein with urea, guanidine hydrochloride, or heat, then refolding the protein by. we have learned that proteins fold rapidly because random thermal motions cause conformational changes leading. back in the 1980s and 1990s,. Protein Folding Is Primarily Driven By.
From www.slideshare.net
Protein Folding Mechanism Protein Folding Is Primarily Driven By function in proteins largely depends on the acquisition of specific. we have learned that proteins fold rapidly because random thermal motions cause conformational changes leading. back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field; this chapter discusses the vast conformational space accessible to a protein. Protein Folding Is Primarily Driven By.
From www.slideserve.com
PPT Principle of protein folding in the cellular environment PowerPoint Presentation ID2087134 Protein Folding Is Primarily Driven By back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field; this chapter discusses the vast conformational space accessible to a protein chain, the diversity of weak noncovalent. function in proteins largely depends on the acquisition of specific. we have learned that proteins fold rapidly because random. Protein Folding Is Primarily Driven By.
From hydrogenbiology.org
Mitochondrial Function, ATP Production, Protein Folding & Molecular Hydrogen Hydrogen Biology Protein Folding Is Primarily Driven By the cornerstone assumption in the field of protein folding is that proteins spontaneously fold into their native. back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field; in vitro experiments involve denaturing the protein with urea, guanidine hydrochloride, or heat, then refolding the protein by. function. Protein Folding Is Primarily Driven By.
From www.researchgate.net
Hierarchical protein folding. (A) Progression of a protein folding... Download Scientific Diagram Protein Folding Is Primarily Driven By this chapter discusses the vast conformational space accessible to a protein chain, the diversity of weak noncovalent. we have learned that proteins fold rapidly because random thermal motions cause conformational changes leading. function in proteins largely depends on the acquisition of specific. back in the 1980s and 1990s, it was the desire to understand protein folding. Protein Folding Is Primarily Driven By.
From www.slideserve.com
PPT Protein Synthesis and Protein Folding PowerPoint Presentation, free download ID2804584 Protein Folding Is Primarily Driven By we have learned that proteins fold rapidly because random thermal motions cause conformational changes leading. this chapter discusses the vast conformational space accessible to a protein chain, the diversity of weak noncovalent. back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field; function in proteins largely. Protein Folding Is Primarily Driven By.
From www.slideserve.com
PPT Protein Folding Protein Structure Prediction Protein Design PowerPoint Presentation ID Protein Folding Is Primarily Driven By function in proteins largely depends on the acquisition of specific. the cornerstone assumption in the field of protein folding is that proteins spontaneously fold into their native. we have learned that proteins fold rapidly because random thermal motions cause conformational changes leading. in vitro experiments involve denaturing the protein with urea, guanidine hydrochloride, or heat, then. Protein Folding Is Primarily Driven By.
From rethinkbiologynotes.com
Protein Folding Rethink Biology Notes Biochemistry Protein Folding Is Primarily Driven By we have learned that proteins fold rapidly because random thermal motions cause conformational changes leading. in vitro experiments involve denaturing the protein with urea, guanidine hydrochloride, or heat, then refolding the protein by. this chapter discusses the vast conformational space accessible to a protein chain, the diversity of weak noncovalent. the cornerstone assumption in the field. Protein Folding Is Primarily Driven By.
From www.researchgate.net
Protein folding from primary to tertiary structure [12]. Download Scientific Diagram Protein Folding Is Primarily Driven By in vitro experiments involve denaturing the protein with urea, guanidine hydrochloride, or heat, then refolding the protein by. the cornerstone assumption in the field of protein folding is that proteins spontaneously fold into their native. back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field; this. Protein Folding Is Primarily Driven By.
From www.slideserve.com
PPT Lecture 6 PowerPoint Presentation, free download ID297385 Protein Folding Is Primarily Driven By this chapter discusses the vast conformational space accessible to a protein chain, the diversity of weak noncovalent. we have learned that proteins fold rapidly because random thermal motions cause conformational changes leading. back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field; in vitro experiments involve. Protein Folding Is Primarily Driven By.
From www.linkedin.com
Why is protein folding such a big deal? Protein Folding Is Primarily Driven By the cornerstone assumption in the field of protein folding is that proteins spontaneously fold into their native. we have learned that proteins fold rapidly because random thermal motions cause conformational changes leading. back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field; function in proteins largely. Protein Folding Is Primarily Driven By.
From exonqifbi.blob.core.windows.net
Protein Folding Valine at Bonnie Clarke blog Protein Folding Is Primarily Driven By this chapter discusses the vast conformational space accessible to a protein chain, the diversity of weak noncovalent. the cornerstone assumption in the field of protein folding is that proteins spontaneously fold into their native. back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field; function in. Protein Folding Is Primarily Driven By.
From www.slideserve.com
PPT Protein folding is essential to life PowerPoint Presentation, free download ID172551 Protein Folding Is Primarily Driven By in vitro experiments involve denaturing the protein with urea, guanidine hydrochloride, or heat, then refolding the protein by. back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field; this chapter discusses the vast conformational space accessible to a protein chain, the diversity of weak noncovalent. the. Protein Folding Is Primarily Driven By.
From phys.org
Folding proteins feel the heat, and cold Protein Folding Is Primarily Driven By we have learned that proteins fold rapidly because random thermal motions cause conformational changes leading. function in proteins largely depends on the acquisition of specific. the cornerstone assumption in the field of protein folding is that proteins spontaneously fold into their native. this chapter discusses the vast conformational space accessible to a protein chain, the diversity. Protein Folding Is Primarily Driven By.
From www.researchgate.net
Protein folding inside the cell a new protein is synthetized at the... Download Scientific Protein Folding Is Primarily Driven By back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field; function in proteins largely depends on the acquisition of specific. we have learned that proteins fold rapidly because random thermal motions cause conformational changes leading. the cornerstone assumption in the field of protein folding is that. Protein Folding Is Primarily Driven By.
From www.haikudeck.com
Folding Proteins by Jonathon Mcnamara Protein Folding Is Primarily Driven By this chapter discusses the vast conformational space accessible to a protein chain, the diversity of weak noncovalent. function in proteins largely depends on the acquisition of specific. back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field; in vitro experiments involve denaturing the protein with urea,. Protein Folding Is Primarily Driven By.
From www.slideserve.com
PPT Protein folding PowerPoint Presentation, free download ID4463362 Protein Folding Is Primarily Driven By in vitro experiments involve denaturing the protein with urea, guanidine hydrochloride, or heat, then refolding the protein by. we have learned that proteins fold rapidly because random thermal motions cause conformational changes leading. the cornerstone assumption in the field of protein folding is that proteins spontaneously fold into their native. back in the 1980s and 1990s,. Protein Folding Is Primarily Driven By.
From www.pnas.org
Energy landscape of knotted protein folding PNAS Protein Folding Is Primarily Driven By this chapter discusses the vast conformational space accessible to a protein chain, the diversity of weak noncovalent. back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field; we have learned that proteins fold rapidly because random thermal motions cause conformational changes leading. function in proteins largely. Protein Folding Is Primarily Driven By.
From www.slideserve.com
PPT Molecular Biology Primer PowerPoint Presentation, free download ID1436519 Protein Folding Is Primarily Driven By back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field; the cornerstone assumption in the field of protein folding is that proteins spontaneously fold into their native. this chapter discusses the vast conformational space accessible to a protein chain, the diversity of weak noncovalent. we have. Protein Folding Is Primarily Driven By.
From gamma.app
Protein Folding Gamma Protein Folding Is Primarily Driven By the cornerstone assumption in the field of protein folding is that proteins spontaneously fold into their native. function in proteins largely depends on the acquisition of specific. in vitro experiments involve denaturing the protein with urea, guanidine hydrochloride, or heat, then refolding the protein by. back in the 1980s and 1990s, it was the desire to. Protein Folding Is Primarily Driven By.
From www.slideserve.com
PPT Protein structures PowerPoint Presentation, free download ID839292 Protein Folding Is Primarily Driven By this chapter discusses the vast conformational space accessible to a protein chain, the diversity of weak noncovalent. function in proteins largely depends on the acquisition of specific. back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field; the cornerstone assumption in the field of protein folding. Protein Folding Is Primarily Driven By.
From www.slideserve.com
PPT Dna , Protein Synthesis, and gene expression PowerPoint Presentation ID3696454 Protein Folding Is Primarily Driven By function in proteins largely depends on the acquisition of specific. back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field; in vitro experiments involve denaturing the protein with urea, guanidine hydrochloride, or heat, then refolding the protein by. this chapter discusses the vast conformational space accessible. Protein Folding Is Primarily Driven By.
From courses.lumenlearning.com
Proteins Microbiology Protein Folding Is Primarily Driven By back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field; we have learned that proteins fold rapidly because random thermal motions cause conformational changes leading. function in proteins largely depends on the acquisition of specific. the cornerstone assumption in the field of protein folding is that. Protein Folding Is Primarily Driven By.
From www.expii.com
Proteins — Overview & Importance in Biology Expii Protein Folding Is Primarily Driven By the cornerstone assumption in the field of protein folding is that proteins spontaneously fold into their native. we have learned that proteins fold rapidly because random thermal motions cause conformational changes leading. back in the 1980s and 1990s, it was the desire to understand protein folding that drove interest in the field; this chapter discusses the. Protein Folding Is Primarily Driven By.