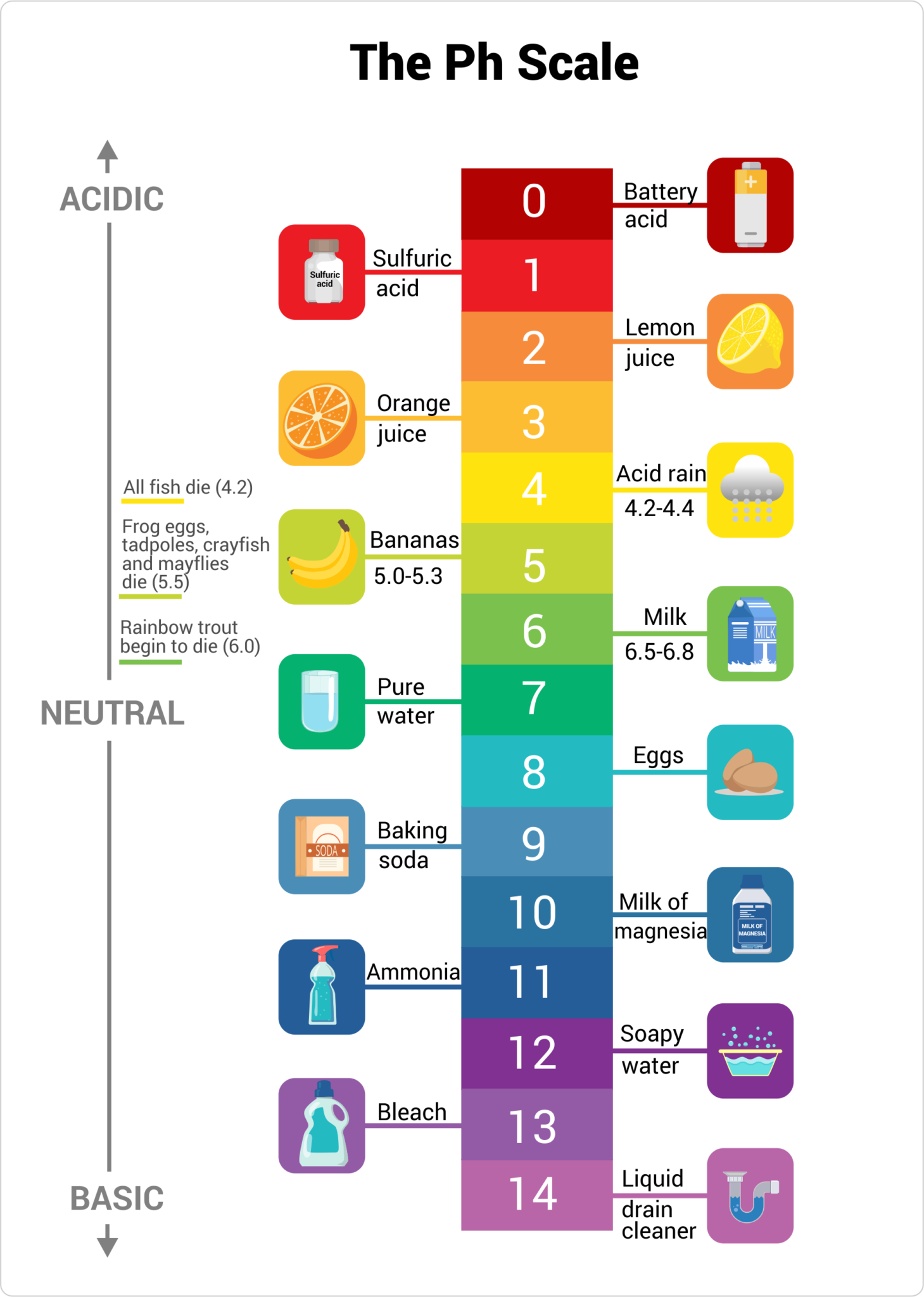
Base Definition Ph Scale . Acidity and alkalinity are measured with a logarithmic scale called ph. To define the ph scale as a measure of acidity of a solution; A strongly acidic solution can have one hundred. Its chemical principle is based on the relative concentration of. Ph is defined as the negative log of hydrogen ion concentration. Tell the origin and the logic of using the ph scale. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. To define the ph scale as a measure of acidity of a solution. It can be used to describe the relative acidity (or basicity) of a solution. Ph is a measure of how acidic or basic a substance is. On this scale, the strongest acid is 0 and the strongest alkali is 14. Apply the same strategy for representing other types of. Because it is based on a logarithmic. Because of its amphoteric nature (i.e., acts as both an acid or a base), water does not always remain as \ (h_2o\) molecules. The ph scale is used to measure the acidity and basicity of solutions of pure water is neutral, acidic and basic.
from www.mometrix.com
The ph scale is used to measure the acidity and basicity of solutions of pure water is neutral, acidic and basic. Apply the same strategy for representing other types of. To define the ph scale as a measure of acidity of a solution. To define the ph scale as a measure of acidity of a solution; A strongly acidic solution can have one hundred. Because of its amphoteric nature (i.e., acts as both an acid or a base), water does not always remain as \ (h_2o\) molecules. Acidity and alkalinity are measured with a logarithmic scale called ph. Because it is based on a logarithmic. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. Ph is defined as the negative log of hydrogen ion concentration.
pH Overview (Chemistry Review Video)
Base Definition Ph Scale Tell the origin and the logic of using the ph scale. Apply the same strategy for representing other types of. Ph is defined as the negative log of hydrogen ion concentration. To define the ph scale as a measure of acidity of a solution; Acidity and alkalinity are measured with a logarithmic scale called ph. Because of its amphoteric nature (i.e., acts as both an acid or a base), water does not always remain as \ (h_2o\) molecules. Ph is a measure of how acidic or basic a substance is. Its chemical principle is based on the relative concentration of. On this scale, the strongest acid is 0 and the strongest alkali is 14. A strongly acidic solution can have one hundred. It can be used to describe the relative acidity (or basicity) of a solution. Tell the origin and the logic of using the ph scale. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. The ph scale is used to measure the acidity and basicity of solutions of pure water is neutral, acidic and basic. Because it is based on a logarithmic. To define the ph scale as a measure of acidity of a solution.
From www.animalia-life.club
Ph Scale Base Definition Ph Scale Acidity and alkalinity are measured with a logarithmic scale called ph. Ph is a measure of how acidic or basic a substance is. The ph scale is used to measure the acidity and basicity of solutions of pure water is neutral, acidic and basic. Because of its amphoteric nature (i.e., acts as both an acid or a base), water does. Base Definition Ph Scale.
From www.chemswot.com
Definition of pH scale in Chemistry Base Definition Ph Scale It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. To define the ph scale as a measure of acidity of a solution; The ph scale is used to measure the acidity and basicity of solutions of pure water is neutral, acidic and basic. Ph is a measure. Base Definition Ph Scale.
From sciencenotes.org
Facts About Acids and Bases Base Definition Ph Scale Tell the origin and the logic of using the ph scale. Apply the same strategy for representing other types of. A strongly acidic solution can have one hundred. The ph scale is used to measure the acidity and basicity of solutions of pure water is neutral, acidic and basic. Ph is defined as the negative log of hydrogen ion concentration.. Base Definition Ph Scale.
From ar.inspiredpencil.com
Basic Ph Scale Base Definition Ph Scale It can be used to describe the relative acidity (or basicity) of a solution. Tell the origin and the logic of using the ph scale. Ph is a measure of how acidic or basic a substance is. To define the ph scale as a measure of acidity of a solution. Because of its amphoteric nature (i.e., acts as both an. Base Definition Ph Scale.
From thebaseisunderasalt.weebly.com
pH Scale THe base is under a salt Base Definition Ph Scale On this scale, the strongest acid is 0 and the strongest alkali is 14. Its chemical principle is based on the relative concentration of. Acidity and alkalinity are measured with a logarithmic scale called ph. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. Tell the origin. Base Definition Ph Scale.
From www.ck12.org
Acids and Bases CK12 Foundation Base Definition Ph Scale Apply the same strategy for representing other types of. Ph is a measure of how acidic or basic a substance is. Its chemical principle is based on the relative concentration of. Ph is defined as the negative log of hydrogen ion concentration. To define the ph scale as a measure of acidity of a solution; A strongly acidic solution can. Base Definition Ph Scale.
From www.youtube.com
MB.1.2. Ph scale (HSC biology) YouTube Base Definition Ph Scale On this scale, the strongest acid is 0 and the strongest alkali is 14. It can be used to describe the relative acidity (or basicity) of a solution. Its chemical principle is based on the relative concentration of. Tell the origin and the logic of using the ph scale. Because it is based on a logarithmic. Acidity and alkalinity are. Base Definition Ph Scale.
From www.pmel.noaa.gov
The pH scale by numbers Base Definition Ph Scale Apply the same strategy for representing other types of. It can be used to describe the relative acidity (or basicity) of a solution. Ph is defined as the negative log of hydrogen ion concentration. Ph is a measure of how acidic or basic a substance is. Because it is based on a logarithmic. A strongly acidic solution can have one. Base Definition Ph Scale.
From acidsandbasesrios.weebly.com
pH Acids and Bases Base Definition Ph Scale Ph is defined as the negative log of hydrogen ion concentration. A strongly acidic solution can have one hundred. Tell the origin and the logic of using the ph scale. On this scale, the strongest acid is 0 and the strongest alkali is 14. Acidity and alkalinity are measured with a logarithmic scale called ph. Apply the same strategy for. Base Definition Ph Scale.
From printablelibmornes.z13.web.core.windows.net
Ph Scale Examples Acids And Bases Base Definition Ph Scale A strongly acidic solution can have one hundred. Ph is defined as the negative log of hydrogen ion concentration. Because of its amphoteric nature (i.e., acts as both an acid or a base), water does not always remain as \ (h_2o\) molecules. To define the ph scale as a measure of acidity of a solution. The ph scale is used. Base Definition Ph Scale.
From sciencenotes.org
The pH Scale of Common Chemicals Base Definition Ph Scale A strongly acidic solution can have one hundred. Because of its amphoteric nature (i.e., acts as both an acid or a base), water does not always remain as \ (h_2o\) molecules. To define the ph scale as a measure of acidity of a solution. Ph is defined as the negative log of hydrogen ion concentration. It can be used to. Base Definition Ph Scale.
From www.vecteezy.com
The ph scale diagram 589313 Vector Art at Vecteezy Base Definition Ph Scale On this scale, the strongest acid is 0 and the strongest alkali is 14. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. Acidity and alkalinity are measured with a logarithmic scale called ph. Because of its amphoteric nature (i.e., acts as both an acid or a. Base Definition Ph Scale.
From www.reddit.com
Reference Guide to Acids vs Bases and the pH Scale r/coolguides Base Definition Ph Scale Its chemical principle is based on the relative concentration of. On this scale, the strongest acid is 0 and the strongest alkali is 14. The ph scale is used to measure the acidity and basicity of solutions of pure water is neutral, acidic and basic. A strongly acidic solution can have one hundred. Tell the origin and the logic of. Base Definition Ph Scale.
From depositphotos.com
Ph scale. infographic acidbase balance. scale for chemical analysis acid base. Stock Vector Base Definition Ph Scale Apply the same strategy for representing other types of. Ph is defined as the negative log of hydrogen ion concentration. Acidity and alkalinity are measured with a logarithmic scale called ph. Because it is based on a logarithmic. To define the ph scale as a measure of acidity of a solution; It takes a numerical value between 0 and 14. Base Definition Ph Scale.
From guitarscalechart.z28.web.core.windows.net
acids and bases ph scale chart 3.12 acids and bases human biology Base Definition Ph Scale A strongly acidic solution can have one hundred. To define the ph scale as a measure of acidity of a solution; Ph is defined as the negative log of hydrogen ion concentration. Because of its amphoteric nature (i.e., acts as both an acid or a base), water does not always remain as \ (h_2o\) molecules. On this scale, the strongest. Base Definition Ph Scale.
From mmerevise.co.uk
Acids and Bases Questions and Revision MME Base Definition Ph Scale Ph is a measure of how acidic or basic a substance is. Because of its amphoteric nature (i.e., acts as both an acid or a base), water does not always remain as \ (h_2o\) molecules. A strongly acidic solution can have one hundred. Acidity and alkalinity are measured with a logarithmic scale called ph. It takes a numerical value between. Base Definition Ph Scale.
From naturalbiohealth.com
How Learning the pH Scale Can Create a More Balanced Diet Natural Bio Health Base Definition Ph Scale A strongly acidic solution can have one hundred. It can be used to describe the relative acidity (or basicity) of a solution. On this scale, the strongest acid is 0 and the strongest alkali is 14. Apply the same strategy for representing other types of. It takes a numerical value between 0 and 14 that measures the relative amount of. Base Definition Ph Scale.
From study.com
Acidic, Basic & Neutral Solutions Overview, pH Scale & Uses Lesson Base Definition Ph Scale Tell the origin and the logic of using the ph scale. A strongly acidic solution can have one hundred. Apply the same strategy for representing other types of. Ph is a measure of how acidic or basic a substance is. Because of its amphoteric nature (i.e., acts as both an acid or a base), water does not always remain as. Base Definition Ph Scale.
From littlebinsforlittlehands.com
Acid, Bases and the pH Scale Little Bins for Little Hands Base Definition Ph Scale A strongly acidic solution can have one hundred. Acidity and alkalinity are measured with a logarithmic scale called ph. Because of its amphoteric nature (i.e., acts as both an acid or a base), water does not always remain as \ (h_2o\) molecules. On this scale, the strongest acid is 0 and the strongest alkali is 14. To define the ph. Base Definition Ph Scale.
From www.oist.jp
pH Values of Common Substances Okinawa Institute of Science and Technology OIST Base Definition Ph Scale Because it is based on a logarithmic. Ph is a measure of how acidic or basic a substance is. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. Apply the same strategy for representing other types of. A strongly acidic solution can have one hundred. It can. Base Definition Ph Scale.
From animalia-life.club
Ph Scale Horizontal Base Definition Ph Scale Tell the origin and the logic of using the ph scale. Because of its amphoteric nature (i.e., acts as both an acid or a base), water does not always remain as \ (h_2o\) molecules. On this scale, the strongest acid is 0 and the strongest alkali is 14. Its chemical principle is based on the relative concentration of. It takes. Base Definition Ph Scale.
From www.alamy.com
Diagram of the pH scale with examples of acidic, neutral and alkaline substances Stock Photo Alamy Base Definition Ph Scale Ph is defined as the negative log of hydrogen ion concentration. On this scale, the strongest acid is 0 and the strongest alkali is 14. Because of its amphoteric nature (i.e., acts as both an acid or a base), water does not always remain as \ (h_2o\) molecules. A strongly acidic solution can have one hundred. Ph is a measure. Base Definition Ph Scale.
From www.narodnatribuna.info
Ph Scale Acids And Bases Examples Base Definition Ph Scale On this scale, the strongest acid is 0 and the strongest alkali is 14. Ph is defined as the negative log of hydrogen ion concentration. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. Apply the same strategy for representing other types of. To define the ph. Base Definition Ph Scale.
From www.mometrix.com
pH Overview (Chemistry Review Video) Base Definition Ph Scale It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. On this scale, the strongest acid is 0 and the strongest alkali is 14. Ph is defined as the negative log of hydrogen ion concentration. Apply the same strategy for representing other types of. Because it is based. Base Definition Ph Scale.
From www.ck12.org
Water, Acids, and Bases CK12 Foundation Base Definition Ph Scale Ph is defined as the negative log of hydrogen ion concentration. On this scale, the strongest acid is 0 and the strongest alkali is 14. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. To define the ph scale as a measure of acidity of a solution;. Base Definition Ph Scale.
From www.youtube.com
PH Scale in Simple Terms YouTube Base Definition Ph Scale To define the ph scale as a measure of acidity of a solution; Because of its amphoteric nature (i.e., acts as both an acid or a base), water does not always remain as \ (h_2o\) molecules. Because it is based on a logarithmic. On this scale, the strongest acid is 0 and the strongest alkali is 14. Tell the origin. Base Definition Ph Scale.
From psiberg.com
The pH Scale of Acid and Bases PSIBERG Base Definition Ph Scale Because of its amphoteric nature (i.e., acts as both an acid or a base), water does not always remain as \ (h_2o\) molecules. It can be used to describe the relative acidity (or basicity) of a solution. Because it is based on a logarithmic. Tell the origin and the logic of using the ph scale. It takes a numerical value. Base Definition Ph Scale.
From www.expii.com
What Is pH? — Definition & Overview Expii Base Definition Ph Scale Ph is a measure of how acidic or basic a substance is. On this scale, the strongest acid is 0 and the strongest alkali is 14. Acidity and alkalinity are measured with a logarithmic scale called ph. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. It. Base Definition Ph Scale.
From preclaboratories.com
Back to Basics Acids, Bases & the pH Scale Precision Laboratories Base Definition Ph Scale On this scale, the strongest acid is 0 and the strongest alkali is 14. It can be used to describe the relative acidity (or basicity) of a solution. Acidity and alkalinity are measured with a logarithmic scale called ph. Ph is a measure of how acidic or basic a substance is. To define the ph scale as a measure of. Base Definition Ph Scale.
From www.teachoo.com
Strength of Acids and Bases (How to find it?) Chemistry Teachoo Base Definition Ph Scale A strongly acidic solution can have one hundred. Apply the same strategy for representing other types of. Tell the origin and the logic of using the ph scale. To define the ph scale as a measure of acidity of a solution; It can be used to describe the relative acidity (or basicity) of a solution. Because it is based on. Base Definition Ph Scale.
From mchsscience.weebly.com
Acids and Bases MCHS Science Base Definition Ph Scale Ph is a measure of how acidic or basic a substance is. Apply the same strategy for representing other types of. The ph scale is used to measure the acidity and basicity of solutions of pure water is neutral, acidic and basic. It can be used to describe the relative acidity (or basicity) of a solution. Because it is based. Base Definition Ph Scale.
From www.toppr.com
pH Definition, Formula, Meaning, Applications and More Base Definition Ph Scale Apply the same strategy for representing other types of. Ph is defined as the negative log of hydrogen ion concentration. The ph scale is used to measure the acidity and basicity of solutions of pure water is neutral, acidic and basic. To define the ph scale as a measure of acidity of a solution. Acidity and alkalinity are measured with. Base Definition Ph Scale.
From www.reagent.co.uk
Who Invented The pH Scale? The Science Blog Base Definition Ph Scale The ph scale is used to measure the acidity and basicity of solutions of pure water is neutral, acidic and basic. Acidity and alkalinity are measured with a logarithmic scale called ph. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. To define the ph scale as. Base Definition Ph Scale.
From amchemistryblog.wordpress.com
4a. Acids and Bases Antonia's chemistry blog Base Definition Ph Scale Tell the origin and the logic of using the ph scale. Because it is based on a logarithmic. A strongly acidic solution can have one hundred. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. Acidity and alkalinity are measured with a logarithmic scale called ph. To. Base Definition Ph Scale.
From nacs.instructure.com
Acids & Bases quest Base Definition Ph Scale It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. Because of its amphoteric nature (i.e., acts as both an acid or a base), water does not always remain as \ (h_2o\) molecules. To define the ph scale as a measure of acidity of a solution. The ph. Base Definition Ph Scale.