How Do Heat Warmers Work . Yet the science behind them is surprisingly straightforward. We're talking about hand warmers! When a hand warmer is exposed to air by unwrapping it or removing its protective covering, oxygen molecules interact with the iron powder, triggering the oxidation. These hotties can reach up to 163. How do hand warmers work? There are five main ingredients inside a hand warmer: Here is everything you need to know about the. By pressing a button in a pouch, which contains a supercooled. A hand warmer contains sodium acetate, dissolved in water. It’s a simple chemical reaction creating heat and keeping you on the ski hills longer. How a hand warmer releases heat. Oxygen in the air reacts with this powder to yield iron oxide—rust—and heat. To activate all warming packs, simply open the pack. The ingredients will begin to heat up as soon as they’re exposed to oxygen (by way of the. Hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation.
from www.powerpal.net
Hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. There are five main ingredients inside a hand warmer: When a hand warmer is exposed to air by unwrapping it or removing its protective covering, oxygen molecules interact with the iron powder, triggering the oxidation. How a hand warmer releases heat. We're talking about hand warmers! How do hand warmers work? By pressing a button in a pouch, which contains a supercooled. Yet the science behind them is surprisingly straightforward. Oxygen in the air reacts with this powder to yield iron oxide—rust—and heat. It’s a simple chemical reaction creating heat and keeping you on the ski hills longer.
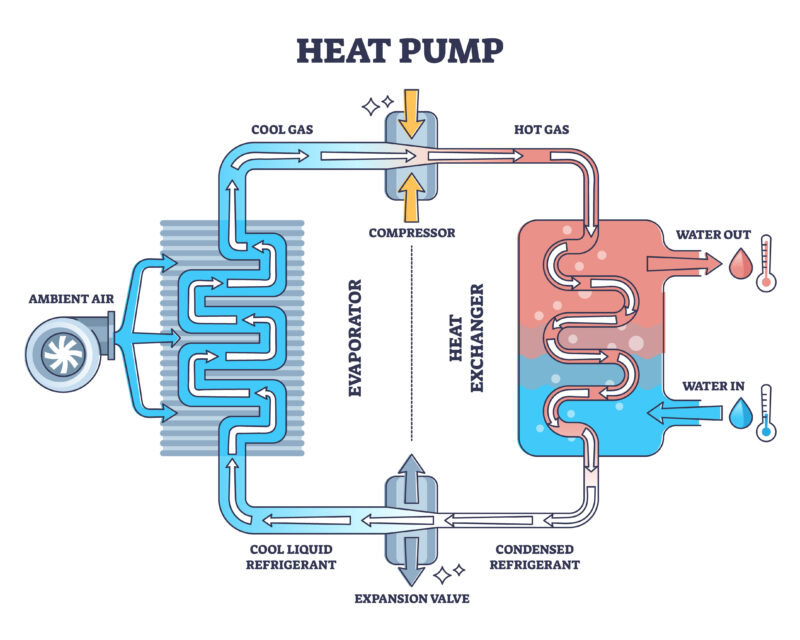
With up to 65 more efficiency, how do heat pumps work? Powerpal
How Do Heat Warmers Work To activate all warming packs, simply open the pack. The ingredients will begin to heat up as soon as they’re exposed to oxygen (by way of the. How do hand warmers work? Oxygen in the air reacts with this powder to yield iron oxide—rust—and heat. When a hand warmer is exposed to air by unwrapping it or removing its protective covering, oxygen molecules interact with the iron powder, triggering the oxidation. Yet the science behind them is surprisingly straightforward. By pressing a button in a pouch, which contains a supercooled. These hotties can reach up to 163. We're talking about hand warmers! It’s a simple chemical reaction creating heat and keeping you on the ski hills longer. Here is everything you need to know about the. To activate all warming packs, simply open the pack. There are five main ingredients inside a hand warmer: How a hand warmer releases heat. A hand warmer contains sodium acetate, dissolved in water. Hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation.
From hiconsumption.com
Primer How Do Hand Warmers Work? HiConsumption How Do Heat Warmers Work We're talking about hand warmers! A hand warmer contains sodium acetate, dissolved in water. These hotties can reach up to 163. How a hand warmer releases heat. To activate all warming packs, simply open the pack. The ingredients will begin to heat up as soon as they’re exposed to oxygen (by way of the. Hand warmers provide a unique and. How Do Heat Warmers Work.
From www.youtube.com
How To Use a Bottle Warmer YouTube How Do Heat Warmers Work How do hand warmers work? The ingredients will begin to heat up as soon as they’re exposed to oxygen (by way of the. It’s a simple chemical reaction creating heat and keeping you on the ski hills longer. Here is everything you need to know about the. To activate all warming packs, simply open the pack. By pressing a button. How Do Heat Warmers Work.
From www.autozone.com
How Does a Car Heater Work? AutoZone How Do Heat Warmers Work The ingredients will begin to heat up as soon as they’re exposed to oxygen (by way of the. We're talking about hand warmers! How do hand warmers work? These hotties can reach up to 163. By pressing a button in a pouch, which contains a supercooled. There are five main ingredients inside a hand warmer: Hand warmers provide a unique. How Do Heat Warmers Work.
From learn.sparkfun.com
Heating Pad Hand Warmer Blanket SparkFun Learn How Do Heat Warmers Work These hotties can reach up to 163. It’s a simple chemical reaction creating heat and keeping you on the ski hills longer. Hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. The ingredients will begin to heat up as soon as they’re exposed to oxygen (by way of the. Oxygen in. How Do Heat Warmers Work.
From ffden-2.phys.uaf.edu
Thermodynamics How Do Heat Warmers Work To activate all warming packs, simply open the pack. How a hand warmer releases heat. A hand warmer contains sodium acetate, dissolved in water. By pressing a button in a pouch, which contains a supercooled. Hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. How do hand warmers work? These hotties. How Do Heat Warmers Work.
From diagrampartsubstratum.z21.web.core.windows.net
Heat Pump Installation Manual How Do Heat Warmers Work The ingredients will begin to heat up as soon as they’re exposed to oxygen (by way of the. To activate all warming packs, simply open the pack. By pressing a button in a pouch, which contains a supercooled. Here is everything you need to know about the. A hand warmer contains sodium acetate, dissolved in water. We're talking about hand. How Do Heat Warmers Work.
From www.got2bwireless.com
How Do Heat Pump Tumble Dryers Work Heat Pumps How Do Heat Warmers Work The ingredients will begin to heat up as soon as they’re exposed to oxygen (by way of the. How a hand warmer releases heat. When a hand warmer is exposed to air by unwrapping it or removing its protective covering, oxygen molecules interact with the iron powder, triggering the oxidation. These hotties can reach up to 163. We're talking about. How Do Heat Warmers Work.
From besthomeheating.com
How Does a Towel Warmer Work? How Do Heat Warmers Work How do hand warmers work? These hotties can reach up to 163. Hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. It’s a simple chemical reaction creating heat and keeping you on the ski hills longer. There are five main ingredients inside a hand warmer: To activate all warming packs, simply. How Do Heat Warmers Work.
From sciencenotes.org
Hand Warmer Chemistry Easy Chemical Hot Packs How Do Heat Warmers Work There are five main ingredients inside a hand warmer: When a hand warmer is exposed to air by unwrapping it or removing its protective covering, oxygen molecules interact with the iron powder, triggering the oxidation. How a hand warmer releases heat. The ingredients will begin to heat up as soon as they’re exposed to oxygen (by way of the. How. How Do Heat Warmers Work.
From sites.google.com
Heat Transfers Solar Cooker Challenge How Do Heat Warmers Work A hand warmer contains sodium acetate, dissolved in water. Oxygen in the air reacts with this powder to yield iron oxide—rust—and heat. To activate all warming packs, simply open the pack. Yet the science behind them is surprisingly straightforward. How do hand warmers work? These hotties can reach up to 163. The ingredients will begin to heat up as soon. How Do Heat Warmers Work.
From www.d-air-conditioning.co.uk
How do Heating and Air Conditioning Units Work? DAir How Do Heat Warmers Work When a hand warmer is exposed to air by unwrapping it or removing its protective covering, oxygen molecules interact with the iron powder, triggering the oxidation. We're talking about hand warmers! The ingredients will begin to heat up as soon as they’re exposed to oxygen (by way of the. Here is everything you need to know about the. It’s a. How Do Heat Warmers Work.
From learnmetrics.com
How Does A Heat Pump Work? StepByStep Heating, Cooling Explained How Do Heat Warmers Work Here is everything you need to know about the. By pressing a button in a pouch, which contains a supercooled. There are five main ingredients inside a hand warmer: A hand warmer contains sodium acetate, dissolved in water. These hotties can reach up to 163. Oxygen in the air reacts with this powder to yield iron oxide—rust—and heat. To activate. How Do Heat Warmers Work.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry How Do Heat Warmers Work To activate all warming packs, simply open the pack. When a hand warmer is exposed to air by unwrapping it or removing its protective covering, oxygen molecules interact with the iron powder, triggering the oxidation. We're talking about hand warmers! How do hand warmers work? By pressing a button in a pouch, which contains a supercooled. Hand warmers provide a. How Do Heat Warmers Work.
From makinghouseswork.cchrc.org
space heating Making Houses Work How Do Heat Warmers Work Here is everything you need to know about the. Oxygen in the air reacts with this powder to yield iron oxide—rust—and heat. There are five main ingredients inside a hand warmer: We're talking about hand warmers! It’s a simple chemical reaction creating heat and keeping you on the ski hills longer. When a hand warmer is exposed to air by. How Do Heat Warmers Work.
From elegant-radiators.co.uk
How Do Towel Warmers Work? How Do Heat Warmers Work To activate all warming packs, simply open the pack. Oxygen in the air reacts with this powder to yield iron oxide—rust—and heat. Here is everything you need to know about the. We're talking about hand warmers! It’s a simple chemical reaction creating heat and keeping you on the ski hills longer. There are five main ingredients inside a hand warmer:. How Do Heat Warmers Work.
From besthomeheating.com
How Does a Towel Warmer Work? How Do Heat Warmers Work By pressing a button in a pouch, which contains a supercooled. The ingredients will begin to heat up as soon as they’re exposed to oxygen (by way of the. How do hand warmers work? Hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. Oxygen in the air reacts with this powder. How Do Heat Warmers Work.
From hiconsumption.com
Primer How Do Hand Warmers Work? HiConsumption How Do Heat Warmers Work By pressing a button in a pouch, which contains a supercooled. Here is everything you need to know about the. How do hand warmers work? The ingredients will begin to heat up as soon as they’re exposed to oxygen (by way of the. When a hand warmer is exposed to air by unwrapping it or removing its protective covering, oxygen. How Do Heat Warmers Work.
From scentgraph.com
What Is A Candle Warmer? Working Mechanisms And Details ScentGraph How Do Heat Warmers Work These hotties can reach up to 163. There are five main ingredients inside a hand warmer: We're talking about hand warmers! Hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. Oxygen in the air reacts with this powder to yield iron oxide—rust—and heat. How a hand warmer releases heat. Here is. How Do Heat Warmers Work.
From www.pinterest.com
How do our warmers work? With simplicity! Pick a warmer add some wax and done. linkinbio How Do Heat Warmers Work It’s a simple chemical reaction creating heat and keeping you on the ski hills longer. To activate all warming packs, simply open the pack. How do hand warmers work? Oxygen in the air reacts with this powder to yield iron oxide—rust—and heat. There are five main ingredients inside a hand warmer: By pressing a button in a pouch, which contains. How Do Heat Warmers Work.
From fyoldmsth.blob.core.windows.net
Heating And Air Conditioning For Residential Buildings at Sharon Steele blog How Do Heat Warmers Work Here is everything you need to know about the. A hand warmer contains sodium acetate, dissolved in water. There are five main ingredients inside a hand warmer: It’s a simple chemical reaction creating heat and keeping you on the ski hills longer. How a hand warmer releases heat. When a hand warmer is exposed to air by unwrapping it or. How Do Heat Warmers Work.
From www.nonothingparty.com
Understanding HVAC How Heating and Cooling Systems Work Best Pick Reports 万博体育主页官网 How Do Heat Warmers Work By pressing a button in a pouch, which contains a supercooled. Yet the science behind them is surprisingly straightforward. It’s a simple chemical reaction creating heat and keeping you on the ski hills longer. How do hand warmers work? Oxygen in the air reacts with this powder to yield iron oxide—rust—and heat. These hotties can reach up to 163. To. How Do Heat Warmers Work.
From www.slideserve.com
PPT Chapter 6 Thermochemistry PowerPoint Presentation, free download ID1549829 How Do Heat Warmers Work It’s a simple chemical reaction creating heat and keeping you on the ski hills longer. A hand warmer contains sodium acetate, dissolved in water. Here is everything you need to know about the. These hotties can reach up to 163. How a hand warmer releases heat. How do hand warmers work? The ingredients will begin to heat up as soon. How Do Heat Warmers Work.
From goclean.masscec.com
How Heat Pump Clothes Dryers Work Clean Energy Lives Here How Do Heat Warmers Work Yet the science behind them is surprisingly straightforward. A hand warmer contains sodium acetate, dissolved in water. It’s a simple chemical reaction creating heat and keeping you on the ski hills longer. Hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. How a hand warmer releases heat. When a hand warmer. How Do Heat Warmers Work.
From ambienteufh.co.uk
How does underfloor heating and cooling work? Ambiente How Do Heat Warmers Work How do hand warmers work? Here is everything you need to know about the. Hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. A hand warmer contains sodium acetate, dissolved in water. To activate all warming packs, simply open the pack. There are five main ingredients inside a hand warmer: When. How Do Heat Warmers Work.
From www.touristsecrets.com
How Do Bottle Warmers Work TouristSecrets How Do Heat Warmers Work How a hand warmer releases heat. Here is everything you need to know about the. We're talking about hand warmers! How do hand warmers work? When a hand warmer is exposed to air by unwrapping it or removing its protective covering, oxygen molecules interact with the iron powder, triggering the oxidation. Hand warmers provide a unique and fun way to. How Do Heat Warmers Work.
From www.lihpao.com
A Comprehensive Guide to Hand Warmers Understanding How They Work and How to Use Them The How Do Heat Warmers Work To activate all warming packs, simply open the pack. There are five main ingredients inside a hand warmer: These hotties can reach up to 163. How do hand warmers work? The ingredients will begin to heat up as soon as they’re exposed to oxygen (by way of the. Oxygen in the air reacts with this powder to yield iron oxide—rust—and. How Do Heat Warmers Work.
From www.hometips.com
How Central Heating Works How Do Heat Warmers Work To activate all warming packs, simply open the pack. Here is everything you need to know about the. By pressing a button in a pouch, which contains a supercooled. When a hand warmer is exposed to air by unwrapping it or removing its protective covering, oxygen molecules interact with the iron powder, triggering the oxidation. How a hand warmer releases. How Do Heat Warmers Work.
From activerain.com
Try this new trend in home heating How Do Heat Warmers Work There are five main ingredients inside a hand warmer: Yet the science behind them is surprisingly straightforward. Hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. By pressing a button in a pouch, which contains a supercooled. We're talking about hand warmers! Oxygen in the air reacts with this powder to. How Do Heat Warmers Work.
From www.youtube.com
Catalytic hand warmer review! YouTube How Do Heat Warmers Work Here is everything you need to know about the. To activate all warming packs, simply open the pack. A hand warmer contains sodium acetate, dissolved in water. When a hand warmer is exposed to air by unwrapping it or removing its protective covering, oxygen molecules interact with the iron powder, triggering the oxidation. It’s a simple chemical reaction creating heat. How Do Heat Warmers Work.
From rockrunner.net
How Do Hand Warmers Work Rockrunner How Do Heat Warmers Work To activate all warming packs, simply open the pack. Here is everything you need to know about the. When a hand warmer is exposed to air by unwrapping it or removing its protective covering, oxygen molecules interact with the iron powder, triggering the oxidation. It’s a simple chemical reaction creating heat and keeping you on the ski hills longer. Yet. How Do Heat Warmers Work.
From watertreatmentservices.co.uk
How Do Heat Pumps Work? How Do Heat Warmers Work By pressing a button in a pouch, which contains a supercooled. A hand warmer contains sodium acetate, dissolved in water. Oxygen in the air reacts with this powder to yield iron oxide—rust—and heat. Here is everything you need to know about the. To activate all warming packs, simply open the pack. When a hand warmer is exposed to air by. How Do Heat Warmers Work.
From www.pinterest.com
How does an element warmer work? This is a photo showing that element warmers use a heating How Do Heat Warmers Work How do hand warmers work? To activate all warming packs, simply open the pack. These hotties can reach up to 163. When a hand warmer is exposed to air by unwrapping it or removing its protective covering, oxygen molecules interact with the iron powder, triggering the oxidation. Hand warmers provide a unique and fun way to study the chemistry of. How Do Heat Warmers Work.
From hotsnapz.com
HotSnapZ Reusable Hand Warmers and Heat Pads How Do Heat Warmers Work To activate all warming packs, simply open the pack. How do hand warmers work? It’s a simple chemical reaction creating heat and keeping you on the ski hills longer. Yet the science behind them is surprisingly straightforward. The ingredients will begin to heat up as soon as they’re exposed to oxygen (by way of the. How a hand warmer releases. How Do Heat Warmers Work.
From www.energystar.gov
ENERGY STAR Ask the Experts Products ENERGY STAR How Do Heat Warmers Work How a hand warmer releases heat. Hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. It’s a simple chemical reaction creating heat and keeping you on the ski hills longer. The ingredients will begin to heat up as soon as they’re exposed to oxygen (by way of the. Here is everything. How Do Heat Warmers Work.
From www.powerpal.net
With up to 65 more efficiency, how do heat pumps work? Powerpal How Do Heat Warmers Work Here is everything you need to know about the. How a hand warmer releases heat. There are five main ingredients inside a hand warmer: To activate all warming packs, simply open the pack. Oxygen in the air reacts with this powder to yield iron oxide—rust—and heat. The ingredients will begin to heat up as soon as they’re exposed to oxygen. How Do Heat Warmers Work.