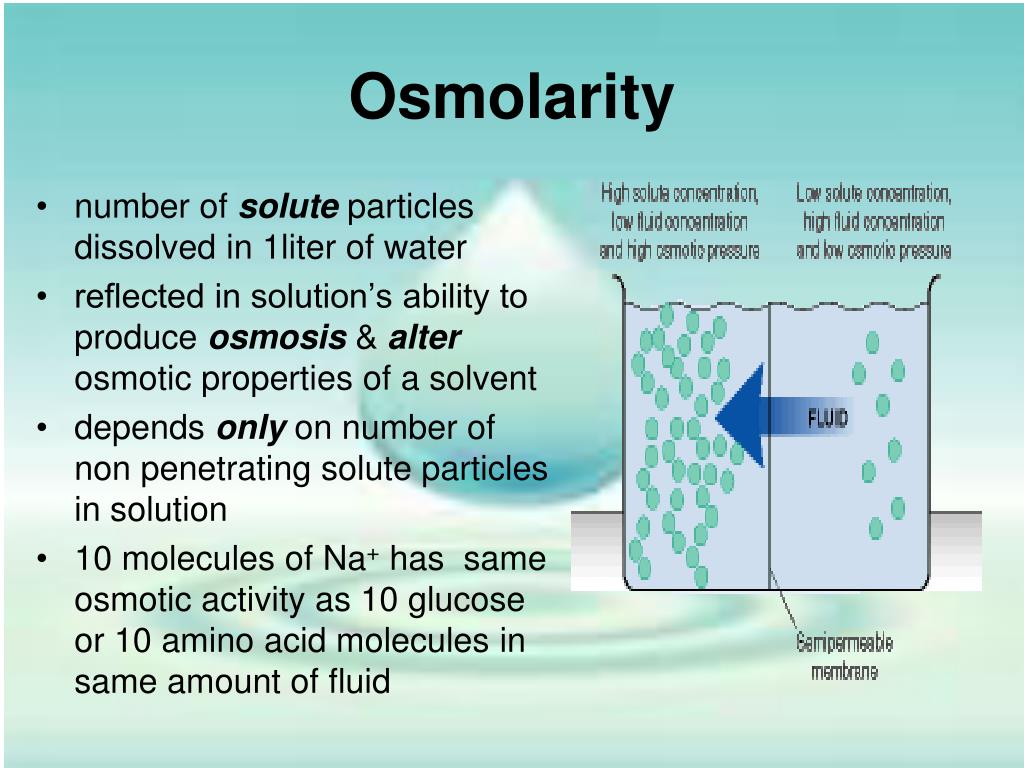
Does Ph Affect Osmolarity . Osmoregulation is the process of maintenance of salt and water balance (osmotic balance) across membranes within the body’s fluids, which are. Osmolality is the concentration of osmoles in a mass of solvent. − ph 4.5 = 100% severe phlebitic. Serum osmolality is affected by the concentration of blood chemicals like chloride, sodium, proteins, bicarbonate, and glucose. − six hour infusions through peripheral vessels: While conductivity and ph have clear value within downstream bioprocessing , osmolality can offer additional information about buffer. 1) rabbit peripheral veins could tolerate the ph (6.46) and the osmolality (779 mosm/kg) of geaa under the. Though similar to molarity, osmolarity refers to the. In biologic systems, osmolality is expressed as mosm/kg. Each of these ions becomes surrounded by solvent molecules, a process called solvation. Animal data isolates traumatic effect of ph and osmolality. These results were as follows: One osmole refers to one mole of osmotically active solute particles. Consequently, an ionic compound contributes more particles to a solution than.
from www.slideserve.com
Serum osmolality is affected by the concentration of blood chemicals like chloride, sodium, proteins, bicarbonate, and glucose. In biologic systems, osmolality is expressed as mosm/kg. While conductivity and ph have clear value within downstream bioprocessing , osmolality can offer additional information about buffer. Each of these ions becomes surrounded by solvent molecules, a process called solvation. Consequently, an ionic compound contributes more particles to a solution than. These results were as follows: − ph 4.5 = 100% severe phlebitic. Though similar to molarity, osmolarity refers to the. Osmoregulation is the process of maintenance of salt and water balance (osmotic balance) across membranes within the body’s fluids, which are. One osmole refers to one mole of osmotically active solute particles.
PPT Fluid & Electrolyte Balance PowerPoint Presentation, free
Does Ph Affect Osmolarity Serum osmolality is affected by the concentration of blood chemicals like chloride, sodium, proteins, bicarbonate, and glucose. Animal data isolates traumatic effect of ph and osmolality. 1) rabbit peripheral veins could tolerate the ph (6.46) and the osmolality (779 mosm/kg) of geaa under the. In biologic systems, osmolality is expressed as mosm/kg. Osmolality is the concentration of osmoles in a mass of solvent. − ph 4.5 = 100% severe phlebitic. While conductivity and ph have clear value within downstream bioprocessing , osmolality can offer additional information about buffer. One osmole refers to one mole of osmotically active solute particles. − six hour infusions through peripheral vessels: Though similar to molarity, osmolarity refers to the. These results were as follows: Consequently, an ionic compound contributes more particles to a solution than. Each of these ions becomes surrounded by solvent molecules, a process called solvation. Osmoregulation is the process of maintenance of salt and water balance (osmotic balance) across membranes within the body’s fluids, which are. Serum osmolality is affected by the concentration of blood chemicals like chloride, sodium, proteins, bicarbonate, and glucose.
From www.slideserve.com
PPT Fluid & Electrolyte Balance PowerPoint Presentation, free Does Ph Affect Osmolarity These results were as follows: In biologic systems, osmolality is expressed as mosm/kg. One osmole refers to one mole of osmotically active solute particles. 1) rabbit peripheral veins could tolerate the ph (6.46) and the osmolality (779 mosm/kg) of geaa under the. Osmolality is the concentration of osmoles in a mass of solvent. Osmoregulation is the process of maintenance of. Does Ph Affect Osmolarity.
From study.com
Serum Osmolality Definition, Calculation & Interpretation Lesson Does Ph Affect Osmolarity Each of these ions becomes surrounded by solvent molecules, a process called solvation. Osmolality is the concentration of osmoles in a mass of solvent. Consequently, an ionic compound contributes more particles to a solution than. Though similar to molarity, osmolarity refers to the. − six hour infusions through peripheral vessels: While conductivity and ph have clear value within downstream bioprocessing. Does Ph Affect Osmolarity.
From www.youtube.com
Osmolality vs Osmolarity (with a mnemonic) Physiology and Chemistry Does Ph Affect Osmolarity Osmoregulation is the process of maintenance of salt and water balance (osmotic balance) across membranes within the body’s fluids, which are. These results were as follows: − ph 4.5 = 100% severe phlebitic. In biologic systems, osmolality is expressed as mosm/kg. While conductivity and ph have clear value within downstream bioprocessing , osmolality can offer additional information about buffer. Each. Does Ph Affect Osmolarity.
From www.youtube.com
Osmolarity Example (Bio) YouTube Does Ph Affect Osmolarity − six hour infusions through peripheral vessels: One osmole refers to one mole of osmotically active solute particles. Osmoregulation is the process of maintenance of salt and water balance (osmotic balance) across membranes within the body’s fluids, which are. These results were as follows: Serum osmolality is affected by the concentration of blood chemicals like chloride, sodium, proteins, bicarbonate, and. Does Ph Affect Osmolarity.
From courses.lumenlearning.com
Osmoregulation and Osmotic Balance Biology II Does Ph Affect Osmolarity − six hour infusions through peripheral vessels: While conductivity and ph have clear value within downstream bioprocessing , osmolality can offer additional information about buffer. Each of these ions becomes surrounded by solvent molecules, a process called solvation. Osmoregulation is the process of maintenance of salt and water balance (osmotic balance) across membranes within the body’s fluids, which are. In. Does Ph Affect Osmolarity.
From dmd.aspetjournals.org
Effect of Osmolality on the Interaction between Apple Does Ph Affect Osmolarity Though similar to molarity, osmolarity refers to the. − ph 4.5 = 100% severe phlebitic. In biologic systems, osmolality is expressed as mosm/kg. While conductivity and ph have clear value within downstream bioprocessing , osmolality can offer additional information about buffer. Each of these ions becomes surrounded by solvent molecules, a process called solvation. Consequently, an ionic compound contributes more. Does Ph Affect Osmolarity.
From www.semanticscholar.org
Figure 4 from Effects of pH and osmolarity on the composition and Does Ph Affect Osmolarity Consequently, an ionic compound contributes more particles to a solution than. − six hour infusions through peripheral vessels: Animal data isolates traumatic effect of ph and osmolality. − ph 4.5 = 100% severe phlebitic. Serum osmolality is affected by the concentration of blood chemicals like chloride, sodium, proteins, bicarbonate, and glucose. While conductivity and ph have clear value within downstream. Does Ph Affect Osmolarity.
From www.rosebook.club
Chapter Nine Regulation of Plasma Osmolality — Channel Your Enthusiasm Does Ph Affect Osmolarity 1) rabbit peripheral veins could tolerate the ph (6.46) and the osmolality (779 mosm/kg) of geaa under the. − ph 4.5 = 100% severe phlebitic. While conductivity and ph have clear value within downstream bioprocessing , osmolality can offer additional information about buffer. Animal data isolates traumatic effect of ph and osmolality. Osmolality is the concentration of osmoles in a. Does Ph Affect Osmolarity.
From captivatebio.com
Understanding pH and Osmolality in Cell Culture Media Captivate Bio Does Ph Affect Osmolarity In biologic systems, osmolality is expressed as mosm/kg. One osmole refers to one mole of osmotically active solute particles. Consequently, an ionic compound contributes more particles to a solution than. Each of these ions becomes surrounded by solvent molecules, a process called solvation. Osmoregulation is the process of maintenance of salt and water balance (osmotic balance) across membranes within the. Does Ph Affect Osmolarity.
From www.slideserve.com
PPT Chapter 6 PowerPoint Presentation, free download ID1710183 Does Ph Affect Osmolarity Osmolality is the concentration of osmoles in a mass of solvent. Each of these ions becomes surrounded by solvent molecules, a process called solvation. One osmole refers to one mole of osmotically active solute particles. In biologic systems, osmolality is expressed as mosm/kg. − six hour infusions through peripheral vessels: Consequently, an ionic compound contributes more particles to a solution. Does Ph Affect Osmolarity.
From www.youtube.com
Chemistry Basics Osmolarity, Osmolality and Tonicity YouTube Does Ph Affect Osmolarity Each of these ions becomes surrounded by solvent molecules, a process called solvation. Serum osmolality is affected by the concentration of blood chemicals like chloride, sodium, proteins, bicarbonate, and glucose. In biologic systems, osmolality is expressed as mosm/kg. 1) rabbit peripheral veins could tolerate the ph (6.46) and the osmolality (779 mosm/kg) of geaa under the. These results were as. Does Ph Affect Osmolarity.
From ditki.com
Biochemistry Glossary Osmosis & Osmolarity 2. Osmolarity ditki Does Ph Affect Osmolarity − ph 4.5 = 100% severe phlebitic. − six hour infusions through peripheral vessels: Osmolality is the concentration of osmoles in a mass of solvent. 1) rabbit peripheral veins could tolerate the ph (6.46) and the osmolality (779 mosm/kg) of geaa under the. Osmoregulation is the process of maintenance of salt and water balance (osmotic balance) across membranes within the. Does Ph Affect Osmolarity.
From exyoqmfwz.blob.core.windows.net
Osmolality Calculated Lower Than Normal at Charles Wolff blog Does Ph Affect Osmolarity In biologic systems, osmolality is expressed as mosm/kg. While conductivity and ph have clear value within downstream bioprocessing , osmolality can offer additional information about buffer. − ph 4.5 = 100% severe phlebitic. Serum osmolality is affected by the concentration of blood chemicals like chloride, sodium, proteins, bicarbonate, and glucose. These results were as follows: Osmolality is the concentration of. Does Ph Affect Osmolarity.
From www.researchgate.net
Effect of osmolarity on the pH i of saponinisolated trophozoites and Does Ph Affect Osmolarity − ph 4.5 = 100% severe phlebitic. Serum osmolality is affected by the concentration of blood chemicals like chloride, sodium, proteins, bicarbonate, and glucose. Each of these ions becomes surrounded by solvent molecules, a process called solvation. − six hour infusions through peripheral vessels: Osmoregulation is the process of maintenance of salt and water balance (osmotic balance) across membranes within. Does Ph Affect Osmolarity.
From exyguffjx.blob.core.windows.net
Osmolarity Formula Pharmacy at Matthew King blog Does Ph Affect Osmolarity Osmolality is the concentration of osmoles in a mass of solvent. − ph 4.5 = 100% severe phlebitic. In biologic systems, osmolality is expressed as mosm/kg. Though similar to molarity, osmolarity refers to the. One osmole refers to one mole of osmotically active solute particles. Animal data isolates traumatic effect of ph and osmolality. These results were as follows: 1). Does Ph Affect Osmolarity.
From www.youtube.com
BIO2020 Episode 024 effects of pH, temp, osmolarity YouTube Does Ph Affect Osmolarity Osmoregulation is the process of maintenance of salt and water balance (osmotic balance) across membranes within the body’s fluids, which are. In biologic systems, osmolality is expressed as mosm/kg. − six hour infusions through peripheral vessels: One osmole refers to one mole of osmotically active solute particles. Animal data isolates traumatic effect of ph and osmolality. Osmolality is the concentration. Does Ph Affect Osmolarity.
From www.sciencefacts.net
Osmosis Definition and How Does it Occur (with Diagram) Does Ph Affect Osmolarity − ph 4.5 = 100% severe phlebitic. Osmolality is the concentration of osmoles in a mass of solvent. Consequently, an ionic compound contributes more particles to a solution than. 1) rabbit peripheral veins could tolerate the ph (6.46) and the osmolality (779 mosm/kg) of geaa under the. Serum osmolality is affected by the concentration of blood chemicals like chloride, sodium,. Does Ph Affect Osmolarity.
From www.slideserve.com
PPT Tonicity, Osmoticity , Osmolarity , & Osmolality PowerPoint Does Ph Affect Osmolarity − ph 4.5 = 100% severe phlebitic. Each of these ions becomes surrounded by solvent molecules, a process called solvation. Serum osmolality is affected by the concentration of blood chemicals like chloride, sodium, proteins, bicarbonate, and glucose. Animal data isolates traumatic effect of ph and osmolality. These results were as follows: 1) rabbit peripheral veins could tolerate the ph (6.46). Does Ph Affect Osmolarity.
From www.youtube.com
Osmosis Osmolarity Osmotic Equilibrium Transport Across the Cell Does Ph Affect Osmolarity Though similar to molarity, osmolarity refers to the. − ph 4.5 = 100% severe phlebitic. Osmolality is the concentration of osmoles in a mass of solvent. While conductivity and ph have clear value within downstream bioprocessing , osmolality can offer additional information about buffer. 1) rabbit peripheral veins could tolerate the ph (6.46) and the osmolality (779 mosm/kg) of geaa. Does Ph Affect Osmolarity.
From www.slideserve.com
PPT Fluids and Electrolytes PowerPoint Presentation, free download Does Ph Affect Osmolarity Serum osmolality is affected by the concentration of blood chemicals like chloride, sodium, proteins, bicarbonate, and glucose. While conductivity and ph have clear value within downstream bioprocessing , osmolality can offer additional information about buffer. Osmolality is the concentration of osmoles in a mass of solvent. These results were as follows: Each of these ions becomes surrounded by solvent molecules,. Does Ph Affect Osmolarity.
From pediaa.com
Difference Between Osmolarity and Osmolality Definition, Explanation Does Ph Affect Osmolarity 1) rabbit peripheral veins could tolerate the ph (6.46) and the osmolality (779 mosm/kg) of geaa under the. While conductivity and ph have clear value within downstream bioprocessing , osmolality can offer additional information about buffer. Osmoregulation is the process of maintenance of salt and water balance (osmotic balance) across membranes within the body’s fluids, which are. − six hour. Does Ph Affect Osmolarity.
From www.slideshare.net
10. Osmoregulation Does Ph Affect Osmolarity − six hour infusions through peripheral vessels: In biologic systems, osmolality is expressed as mosm/kg. These results were as follows: While conductivity and ph have clear value within downstream bioprocessing , osmolality can offer additional information about buffer. Though similar to molarity, osmolarity refers to the. Serum osmolality is affected by the concentration of blood chemicals like chloride, sodium, proteins,. Does Ph Affect Osmolarity.
From studylib.net
Understanding pH and Osmolarity Does Ph Affect Osmolarity One osmole refers to one mole of osmotically active solute particles. − six hour infusions through peripheral vessels: 1) rabbit peripheral veins could tolerate the ph (6.46) and the osmolality (779 mosm/kg) of geaa under the. Consequently, an ionic compound contributes more particles to a solution than. Animal data isolates traumatic effect of ph and osmolality. Each of these ions. Does Ph Affect Osmolarity.
From drawittoknowit.com
Anatomy & Physiology Osmosis and Osmolarity ditki medical Does Ph Affect Osmolarity − ph 4.5 = 100% severe phlebitic. Consequently, an ionic compound contributes more particles to a solution than. Each of these ions becomes surrounded by solvent molecules, a process called solvation. Animal data isolates traumatic effect of ph and osmolality. While conductivity and ph have clear value within downstream bioprocessing , osmolality can offer additional information about buffer. These results. Does Ph Affect Osmolarity.
From www.slideserve.com
PPT Osmolality (calc) = 2 x Na + glucose + urea **if all measurements Does Ph Affect Osmolarity Serum osmolality is affected by the concentration of blood chemicals like chloride, sodium, proteins, bicarbonate, and glucose. Each of these ions becomes surrounded by solvent molecules, a process called solvation. − ph 4.5 = 100% severe phlebitic. Animal data isolates traumatic effect of ph and osmolality. − six hour infusions through peripheral vessels: Though similar to molarity, osmolarity refers to. Does Ph Affect Osmolarity.
From www.slideshare.net
Urinalysis 3/27 Does Ph Affect Osmolarity While conductivity and ph have clear value within downstream bioprocessing , osmolality can offer additional information about buffer. Consequently, an ionic compound contributes more particles to a solution than. These results were as follows: 1) rabbit peripheral veins could tolerate the ph (6.46) and the osmolality (779 mosm/kg) of geaa under the. Osmolality is the concentration of osmoles in a. Does Ph Affect Osmolarity.
From basicmedicalkey.com
Control of Body Fluid Osmolality and Volume Basicmedical Key Does Ph Affect Osmolarity − ph 4.5 = 100% severe phlebitic. Osmoregulation is the process of maintenance of salt and water balance (osmotic balance) across membranes within the body’s fluids, which are. In biologic systems, osmolality is expressed as mosm/kg. Consequently, an ionic compound contributes more particles to a solution than. These results were as follows: Each of these ions becomes surrounded by solvent. Does Ph Affect Osmolarity.
From blog.healthmatters.io
What is Osmolality? HealthMatters.io Lab results explained Does Ph Affect Osmolarity One osmole refers to one mole of osmotically active solute particles. 1) rabbit peripheral veins could tolerate the ph (6.46) and the osmolality (779 mosm/kg) of geaa under the. Osmolality is the concentration of osmoles in a mass of solvent. − six hour infusions through peripheral vessels: Though similar to molarity, osmolarity refers to the. Consequently, an ionic compound contributes. Does Ph Affect Osmolarity.
From ditki.com
Biochemistry Glossary Osmosis & Osmolarity 1. Osmosis ditki medical Does Ph Affect Osmolarity − six hour infusions through peripheral vessels: Serum osmolality is affected by the concentration of blood chemicals like chloride, sodium, proteins, bicarbonate, and glucose. Consequently, an ionic compound contributes more particles to a solution than. 1) rabbit peripheral veins could tolerate the ph (6.46) and the osmolality (779 mosm/kg) of geaa under the. One osmole refers to one mole of. Does Ph Affect Osmolarity.
From biologydictionary.net
Osmolarity The Definitive Guide Biology Dictionary Does Ph Affect Osmolarity While conductivity and ph have clear value within downstream bioprocessing , osmolality can offer additional information about buffer. One osmole refers to one mole of osmotically active solute particles. − six hour infusions through peripheral vessels: Serum osmolality is affected by the concentration of blood chemicals like chloride, sodium, proteins, bicarbonate, and glucose. 1) rabbit peripheral veins could tolerate the. Does Ph Affect Osmolarity.
From loeckbdwf.blob.core.windows.net
Significance Of Osmolality Lab Test at Terry Wray blog Does Ph Affect Osmolarity Though similar to molarity, osmolarity refers to the. − ph 4.5 = 100% severe phlebitic. One osmole refers to one mole of osmotically active solute particles. Animal data isolates traumatic effect of ph and osmolality. In biologic systems, osmolality is expressed as mosm/kg. − six hour infusions through peripheral vessels: Consequently, an ionic compound contributes more particles to a solution. Does Ph Affect Osmolarity.
From droualb.faculty.mjc.edu
Chapter 4 Does Ph Affect Osmolarity Serum osmolality is affected by the concentration of blood chemicals like chloride, sodium, proteins, bicarbonate, and glucose. − six hour infusions through peripheral vessels: 1) rabbit peripheral veins could tolerate the ph (6.46) and the osmolality (779 mosm/kg) of geaa under the. Each of these ions becomes surrounded by solvent molecules, a process called solvation. Osmolality is the concentration of. Does Ph Affect Osmolarity.
From lookfordiagnosis.com
Osmolar Concentration; Ionic Strength; Osmolarity; Osmolality Does Ph Affect Osmolarity Consequently, an ionic compound contributes more particles to a solution than. − ph 4.5 = 100% severe phlebitic. Osmoregulation is the process of maintenance of salt and water balance (osmotic balance) across membranes within the body’s fluids, which are. In biologic systems, osmolality is expressed as mosm/kg. Though similar to molarity, osmolarity refers to the. Animal data isolates traumatic effect. Does Ph Affect Osmolarity.
From www.researchgate.net
Effect of osmolality and pH on functional membrane integrity (a, b) and Does Ph Affect Osmolarity In biologic systems, osmolality is expressed as mosm/kg. Animal data isolates traumatic effect of ph and osmolality. Osmoregulation is the process of maintenance of salt and water balance (osmotic balance) across membranes within the body’s fluids, which are. − ph 4.5 = 100% severe phlebitic. While conductivity and ph have clear value within downstream bioprocessing , osmolality can offer additional. Does Ph Affect Osmolarity.
From www.slideserve.com
PPT Osmoregulation PowerPoint Presentation, free download ID5609639 Does Ph Affect Osmolarity Osmolality is the concentration of osmoles in a mass of solvent. Consequently, an ionic compound contributes more particles to a solution than. These results were as follows: In biologic systems, osmolality is expressed as mosm/kg. Though similar to molarity, osmolarity refers to the. Osmoregulation is the process of maintenance of salt and water balance (osmotic balance) across membranes within the. Does Ph Affect Osmolarity.