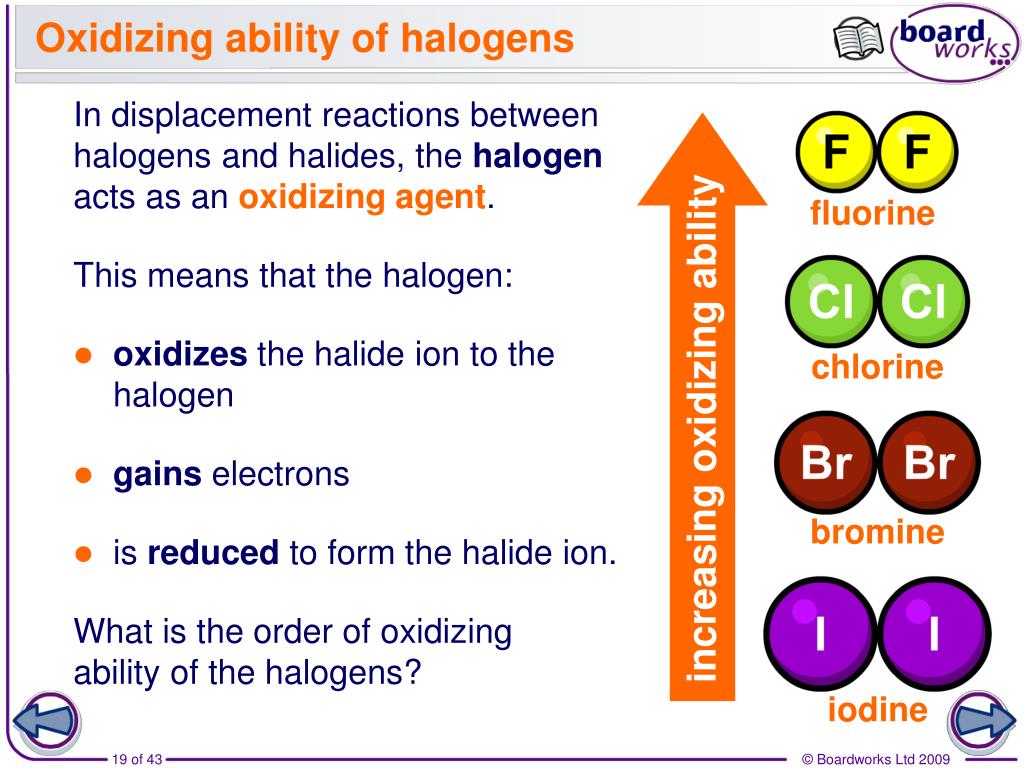
Bromine + Potassium Fluoride . Bromine trifluoride is a strong fluorinating agent that is able to convert a metal (e.g., vanadium) to its associated fluoride compound, (i.e., vf 5). The most common electrolysis procedure is to use a molten mixture. What results might you get if fluorine water and potassium fluoride were in the experiment too? The slideshow shows what happens when solutions of chlorine, bromine and iodine are added to various potassium halide close halide a halide ion is an. Write word and symbol equations for each of the. The major method for preparing fluorine is electrolytic oxidation. Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Potassium bromide is held together by ionic bonds. The potassium has given 1 electron to the bromine, which results in both of the atoms having full outer shells. As bromine received an electron, it will have.
from www.slideserve.com
The slideshow shows what happens when solutions of chlorine, bromine and iodine are added to various potassium halide close halide a halide ion is an. What results might you get if fluorine water and potassium fluoride were in the experiment too? Write word and symbol equations for each of the. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Bromine trifluoride is a strong fluorinating agent that is able to convert a metal (e.g., vanadium) to its associated fluoride compound, (i.e., vf 5). The potassium has given 1 electron to the bromine, which results in both of the atoms having full outer shells. Potassium bromide is held together by ionic bonds. The major method for preparing fluorine is electrolytic oxidation. The most common electrolysis procedure is to use a molten mixture. As bromine received an electron, it will have.
PPT Halogens PowerPoint Presentation, free download ID2110165
Bromine + Potassium Fluoride Potassium bromide is held together by ionic bonds. What results might you get if fluorine water and potassium fluoride were in the experiment too? The most common electrolysis procedure is to use a molten mixture. Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. The slideshow shows what happens when solutions of chlorine, bromine and iodine are added to various potassium halide close halide a halide ion is an. The major method for preparing fluorine is electrolytic oxidation. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Write word and symbol equations for each of the. Bromine trifluoride is a strong fluorinating agent that is able to convert a metal (e.g., vanadium) to its associated fluoride compound, (i.e., vf 5). As bromine received an electron, it will have. The potassium has given 1 electron to the bromine, which results in both of the atoms having full outer shells. Potassium bromide is held together by ionic bonds.
From www.desertcart.in
Buy Focus On 100 Most Popular Oxidizing Agents Oxidizing Agent Bromine + Potassium Fluoride As bromine received an electron, it will have. The potassium has given 1 electron to the bromine, which results in both of the atoms having full outer shells. The slideshow shows what happens when solutions of chlorine, bromine and iodine are added to various potassium halide close halide a halide ion is an. The major method for preparing fluorine is. Bromine + Potassium Fluoride.
From brainly.in
Reaction between sodium iodide and bromine water Brainly.in Bromine + Potassium Fluoride Write word and symbol equations for each of the. The potassium has given 1 electron to the bromine, which results in both of the atoms having full outer shells. Potassium bromide is held together by ionic bonds. The slideshow shows what happens when solutions of chlorine, bromine and iodine are added to various potassium halide close halide a halide ion. Bromine + Potassium Fluoride.
From www.numerade.com
SOLVED Arrange these bonds in order of increasing ionic character Bromine + Potassium Fluoride As bromine received an electron, it will have. The potassium has given 1 electron to the bromine, which results in both of the atoms having full outer shells. Write word and symbol equations for each of the. The most common electrolysis procedure is to use a molten mixture. The slideshow shows what happens when solutions of chlorine, bromine and iodine. Bromine + Potassium Fluoride.
From hamptonresearch.com
Hampton Research Bromine + Potassium Fluoride What results might you get if fluorine water and potassium fluoride were in the experiment too? The major method for preparing fluorine is electrolytic oxidation. Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. Bromine trifluoride is a strong fluorinating agent that is able to convert a metal (e.g.,. Bromine + Potassium Fluoride.
From www.youtube.com
Net ionic equation hydrochloric acid and potassium fluoride YouTube Bromine + Potassium Fluoride If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: What results might you get if fluorine water and potassium fluoride were in the experiment too? Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. The slideshow shows what happens when solutions. Bromine + Potassium Fluoride.
From www.slideshare.net
Chemical Reactions Bromine + Potassium Fluoride Write word and symbol equations for each of the. The slideshow shows what happens when solutions of chlorine, bromine and iodine are added to various potassium halide close halide a halide ion is an. Potassium bromide is held together by ionic bonds. The potassium has given 1 electron to the bromine, which results in both of the atoms having full. Bromine + Potassium Fluoride.
From cesfmvkc.blob.core.windows.net
Liquid Bromine Is Added To A Solution Of Potassium Iodide at Steven Bromine + Potassium Fluoride The slideshow shows what happens when solutions of chlorine, bromine and iodine are added to various potassium halide close halide a halide ion is an. As bromine received an electron, it will have. What results might you get if fluorine water and potassium fluoride were in the experiment too? Potassium bromide is held together by ionic bonds. If you add. Bromine + Potassium Fluoride.
From slideplayer.com
Stoichiometry Unit 8 Lesson ppt download Bromine + Potassium Fluoride Bromine trifluoride is a strong fluorinating agent that is able to convert a metal (e.g., vanadium) to its associated fluoride compound, (i.e., vf 5). What results might you get if fluorine water and potassium fluoride were in the experiment too? As bromine received an electron, it will have. The most common electrolysis procedure is to use a molten mixture. The. Bromine + Potassium Fluoride.
From www.youtube.com
Potassium forms a highly ionic compoundhen it combines with a. Chlorine Bromine + Potassium Fluoride What results might you get if fluorine water and potassium fluoride were in the experiment too? The major method for preparing fluorine is electrolytic oxidation. The potassium has given 1 electron to the bromine, which results in both of the atoms having full outer shells. The most common electrolysis procedure is to use a molten mixture. Write word and symbol. Bromine + Potassium Fluoride.
From fphoto.photoshelter.com
science chemistry redox reaction magnesium hydrochloric acid Bromine + Potassium Fluoride What results might you get if fluorine water and potassium fluoride were in the experiment too? As bromine received an electron, it will have. The major method for preparing fluorine is electrolytic oxidation. Potassium bromide is held together by ionic bonds. The potassium has given 1 electron to the bromine, which results in both of the atoms having full outer. Bromine + Potassium Fluoride.
From www.alamy.com
BrF3 bromine trifluoride CAS 7787715 chemical substance in white Bromine + Potassium Fluoride As bromine received an electron, it will have. The slideshow shows what happens when solutions of chlorine, bromine and iodine are added to various potassium halide close halide a halide ion is an. The major method for preparing fluorine is electrolytic oxidation. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Write. Bromine + Potassium Fluoride.
From www.numerade.com
SOLVED Potassium bromide + chlorine = potassium chloride + bromine Bromine + Potassium Fluoride The most common electrolysis procedure is to use a molten mixture. The potassium has given 1 electron to the bromine, which results in both of the atoms having full outer shells. The major method for preparing fluorine is electrolytic oxidation. Potassium bromide is held together by ionic bonds. As bromine received an electron, it will have. Write word and symbol. Bromine + Potassium Fluoride.
From www.myxxgirl.com
How To Balance Ki Br Kbr I Potassium Iodide My XXX Hot Girl Bromine + Potassium Fluoride Write word and symbol equations for each of the. The slideshow shows what happens when solutions of chlorine, bromine and iodine are added to various potassium halide close halide a halide ion is an. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: The most common electrolysis procedure is to use a. Bromine + Potassium Fluoride.
From joiozxivu.blob.core.windows.net
Bromine Color Solution at Sharon Duran blog Bromine + Potassium Fluoride The slideshow shows what happens when solutions of chlorine, bromine and iodine are added to various potassium halide close halide a halide ion is an. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from. Bromine + Potassium Fluoride.
From dxohmtoii.blob.core.windows.net
Lewis Dot Structure For Calcium Chlorine at Tony Hammon blog Bromine + Potassium Fluoride Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. The most common electrolysis procedure is to use a molten mixture. What results might you get if fluorine water and potassium fluoride were in the experiment too? If you add chlorine solution to colourless potassium bromide or potassium iodide solution. Bromine + Potassium Fluoride.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2110165 Bromine + Potassium Fluoride The potassium has given 1 electron to the bromine, which results in both of the atoms having full outer shells. Write word and symbol equations for each of the. As bromine received an electron, it will have. The most common electrolysis procedure is to use a molten mixture. Bromine only displaces iodine from potassium iodide and the least reactive iodine. Bromine + Potassium Fluoride.
From periodictable.me
Bromine Valence Electrons Bromine Valency (Br) Dot Diagram Bromine + Potassium Fluoride The potassium has given 1 electron to the bromine, which results in both of the atoms having full outer shells. The most common electrolysis procedure is to use a molten mixture. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: The major method for preparing fluorine is electrolytic oxidation. As bromine received. Bromine + Potassium Fluoride.
From www.bulkreefsupply.com
Potassium Iodide Fluoride Concentrate Bulk Reef Supply Bromine + Potassium Fluoride The major method for preparing fluorine is electrolytic oxidation. Write word and symbol equations for each of the. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: The most common electrolysis procedure is to use a molten mixture. As bromine received an electron, it will have. Bromine only displaces iodine from potassium. Bromine + Potassium Fluoride.
From www.thesciencehive.co.uk
Group7halogensanswers — the science sauce Bromine + Potassium Fluoride The most common electrolysis procedure is to use a molten mixture. The potassium has given 1 electron to the bromine, which results in both of the atoms having full outer shells. The major method for preparing fluorine is electrolytic oxidation. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Bromine only displaces. Bromine + Potassium Fluoride.
From klaalfdoh.blob.core.windows.net
Lead (Ii) Nitrate Reacts With Sodium Phosphate at James McCord blog Bromine + Potassium Fluoride Write word and symbol equations for each of the. Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. As bromine received an electron, it will have. The most common electrolysis procedure is to use a molten mixture. What results might you get if fluorine water and potassium fluoride were. Bromine + Potassium Fluoride.
From www.youtube.com
How to Write the Formula for Cobalt (III) bromide YouTube Bromine + Potassium Fluoride The slideshow shows what happens when solutions of chlorine, bromine and iodine are added to various potassium halide close halide a halide ion is an. The most common electrolysis procedure is to use a molten mixture. What results might you get if fluorine water and potassium fluoride were in the experiment too? Bromine only displaces iodine from potassium iodide and. Bromine + Potassium Fluoride.
From www.tradeindia.com
Potassium Fluoride Application Pharmaceutical Industry at Best Price Bromine + Potassium Fluoride Write word and symbol equations for each of the. Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: The slideshow shows what happens when solutions of chlorine, bromine and iodine are added. Bromine + Potassium Fluoride.
From cesfmvkc.blob.core.windows.net
Liquid Bromine Is Added To A Solution Of Potassium Iodide at Steven Bromine + Potassium Fluoride The slideshow shows what happens when solutions of chlorine, bromine and iodine are added to various potassium halide close halide a halide ion is an. The major method for preparing fluorine is electrolytic oxidation. As bromine received an electron, it will have. Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from. Bromine + Potassium Fluoride.
From www.numerade.com
SOLVEDWrite the formula for each of the following simple binary ionic Bromine + Potassium Fluoride What results might you get if fluorine water and potassium fluoride were in the experiment too? The major method for preparing fluorine is electrolytic oxidation. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Write word and symbol equations for each of the. The potassium has given 1 electron to the bromine,. Bromine + Potassium Fluoride.
From joieqcwyg.blob.core.windows.net
Bromine Plus Ion at David Martinelli blog Bromine + Potassium Fluoride What results might you get if fluorine water and potassium fluoride were in the experiment too? Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. Potassium bromide is held together by ionic bonds. Bromine trifluoride is a strong fluorinating agent that is able to convert a metal (e.g., vanadium). Bromine + Potassium Fluoride.
From slideplayer.com
Unit E Chemical Bonding ppt download Bromine + Potassium Fluoride Bromine trifluoride is a strong fluorinating agent that is able to convert a metal (e.g., vanadium) to its associated fluoride compound, (i.e., vf 5). If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: The most common electrolysis procedure is to use a molten mixture. What results might you get if fluorine water. Bromine + Potassium Fluoride.
From fphoto.photoshelter.com
science chemistry halide displacement reaction chlorine postassium Bromine + Potassium Fluoride As bromine received an electron, it will have. What results might you get if fluorine water and potassium fluoride were in the experiment too? If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their. Bromine + Potassium Fluoride.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2110165 Bromine + Potassium Fluoride The slideshow shows what happens when solutions of chlorine, bromine and iodine are added to various potassium halide close halide a halide ion is an. As bromine received an electron, it will have. Potassium bromide is held together by ionic bonds. What results might you get if fluorine water and potassium fluoride were in the experiment too? The major method. Bromine + Potassium Fluoride.
From www.numerade.com
SOLVED 7) Potassium Bromine 8) Potassium + Oxygen Formula Formula Bromine + Potassium Fluoride What results might you get if fluorine water and potassium fluoride were in the experiment too? The potassium has given 1 electron to the bromine, which results in both of the atoms having full outer shells. Write word and symbol equations for each of the. The most common electrolysis procedure is to use a molten mixture. As bromine received an. Bromine + Potassium Fluoride.
From www.numerade.com
SOLVEDArrange the following bonds in order of increasing ionic Bromine + Potassium Fluoride The slideshow shows what happens when solutions of chlorine, bromine and iodine are added to various potassium halide close halide a halide ion is an. As bromine received an electron, it will have. Write word and symbol equations for each of the. Bromine trifluoride is a strong fluorinating agent that is able to convert a metal (e.g., vanadium) to its. Bromine + Potassium Fluoride.
From stock.adobe.com
Diagram explaining Atomic Radius using diatomic molecules. Oxygen Bromine + Potassium Fluoride Potassium bromide is held together by ionic bonds. The major method for preparing fluorine is electrolytic oxidation. The potassium has given 1 electron to the bromine, which results in both of the atoms having full outer shells. Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. Bromine trifluoride is. Bromine + Potassium Fluoride.
From bestonlinecollegesdegrees.com
Agarose Gel Recipe Ethidium Bromide Besto Blog Bromine + Potassium Fluoride The major method for preparing fluorine is electrolytic oxidation. The most common electrolysis procedure is to use a molten mixture. What results might you get if fluorine water and potassium fluoride were in the experiment too? Potassium bromide is held together by ionic bonds. Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or. Bromine + Potassium Fluoride.
From www.indiamart.com
Potassium Fluoride, For Industrial, Packaging Size 50 Kg at Rs 230/kg Bromine + Potassium Fluoride Write word and symbol equations for each of the. Potassium bromide is held together by ionic bonds. As bromine received an electron, it will have. The most common electrolysis procedure is to use a molten mixture. The potassium has given 1 electron to the bromine, which results in both of the atoms having full outer shells. Bromine trifluoride is a. Bromine + Potassium Fluoride.
From socratic.org
What is the balanced chemical equation for this reaction Gaseous Bromine + Potassium Fluoride Bromine trifluoride is a strong fluorinating agent that is able to convert a metal (e.g., vanadium) to its associated fluoride compound, (i.e., vf 5). Potassium bromide is held together by ionic bonds. What results might you get if fluorine water and potassium fluoride were in the experiment too? The slideshow shows what happens when solutions of chlorine, bromine and iodine. Bromine + Potassium Fluoride.
From aluminumgenjin.blogspot.com
Aluminum Aluminum Bromide Bromine + Potassium Fluoride If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: The slideshow shows what happens when solutions of chlorine, bromine and iodine are added to various potassium halide close halide a halide ion is an. The major method for preparing fluorine is electrolytic oxidation. Potassium bromide is held together by ionic bonds. The. Bromine + Potassium Fluoride.