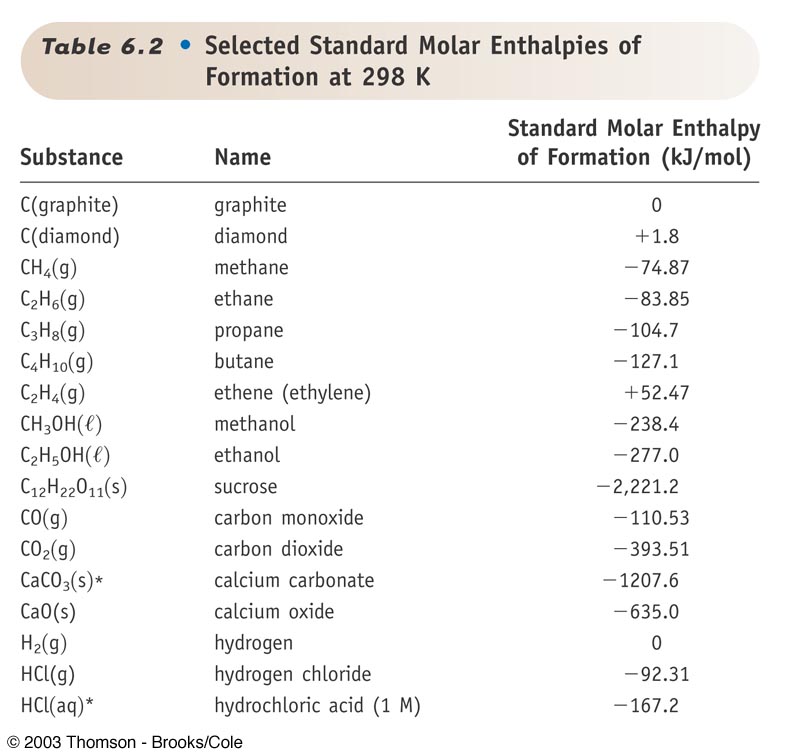
Standard Enthalpy Of Formation H2O Liquid . Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of pressure and 298.15. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. All standard state, 25 °c and 1 bar (written to 1 decimal place). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure.
from rayb78.github.io
All standard state, 25 °c and 1 bar (written to 1 decimal place). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of pressure and 298.15. Mallard, eds, nist chemistry webbook, nist standard reference database. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen.
Heat Of Formation Chart
Standard Enthalpy Of Formation H2O Liquid Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of pressure and 298.15. Mallard, eds, nist chemistry webbook, nist standard reference database. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation H2O Liquid Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Mallard, eds, nist chemistry webbook,. Standard Enthalpy Of Formation H2O Liquid.
From www.chegg.com
Use standard enthalpies of formation to calculate Standard Enthalpy Of Formation H2O Liquid All standard state, 25 °c and 1 bar (written to 1 decimal place). This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Mallard,. Standard Enthalpy Of Formation H2O Liquid.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Enthalpy Of Formation H2O Liquid All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of pressure and 298.15. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole. Standard Enthalpy Of Formation H2O Liquid.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation H2O Liquid Mallard, eds, nist chemistry webbook, nist standard reference database. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of pressure and 298.15.. Standard Enthalpy Of Formation H2O Liquid.
From engineering.stackexchange.com
mechanical engineering Difficulty in understand the meaning of Standard Enthalpy Of Formation H2O Liquid 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. Mallard, eds, nist chemistry. Standard Enthalpy Of Formation H2O Liquid.
From www.toppr.com
The standard enthalpies of formation of CO2(g), H2O(l) and glucose (s Standard Enthalpy Of Formation H2O Liquid This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The standard enthalpy of formation,. Standard Enthalpy Of Formation H2O Liquid.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of reaction 2H2(g Standard Enthalpy Of Formation H2O Liquid Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. Mallard, eds, nist chemistry webbook, nist standard reference database. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. Standard Enthalpy Of Formation H2O Liquid.
From www.youtube.com
The enthalpy of combustion of CH4(g) when H2O (l) is formed YouTube Standard Enthalpy Of Formation H2O Liquid 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. All standard state, 25 °c and 1 bar (written to 1 decimal place). Mallard, eds, nist chemistry webbook, nist standard reference database. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of. Standard Enthalpy Of Formation H2O Liquid.
From stahonorschemistry.weebly.com
III Calculating Enthalpies STA Form IV Honors Chemistry Standard Enthalpy Of Formation H2O Liquid 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. All standard state, 25 °c and 1 bar (written to 1 decimal place). This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Mallard,. Standard Enthalpy Of Formation H2O Liquid.
From joilylugg.blob.core.windows.net
Standard Enthalpy Of Formation Def at Sandra Leonard blog Standard Enthalpy Of Formation H2O Liquid The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of pressure and. Standard Enthalpy Of Formation H2O Liquid.
From www.bartleby.com
Answered Using the standard enthalpies of… bartleby Standard Enthalpy Of Formation H2O Liquid Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. Mallard, eds, nist chemistry webbook, nist standard reference database. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. Standard Enthalpy Of Formation H2O Liquid.
From haipernews.com
How To Calculate Heat Of Haiper Standard Enthalpy Of Formation H2O Liquid Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. 193 rows in chemistry and. Standard Enthalpy Of Formation H2O Liquid.
From www.numerade.com
SOLVED Enthalpy of formation of phenol (C6H5OH) is 165 kJ/mol , and Standard Enthalpy Of Formation H2O Liquid Mallard, eds, nist chemistry webbook, nist standard reference database. All standard state, 25 °c and 1 bar (written to 1 decimal place). Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The standard enthalpy of formation is. Standard Enthalpy Of Formation H2O Liquid.
From storage.googleapis.com
Standard Enthalpy Of Formation Sulfuric Acid Standard Enthalpy Of Formation H2O Liquid The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. This table lists the. Standard Enthalpy Of Formation H2O Liquid.
From cepbtpfh.blob.core.windows.net
Standard Heat Of Formation Hydrogen Peroxide at John Ahmed blog Standard Enthalpy Of Formation H2O Liquid Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation. Standard Enthalpy Of Formation H2O Liquid.
From schoolworkhelper.net
Standard Enthalpies of Formation, ΔH°f SchoolWorkHelper Standard Enthalpy Of Formation H2O Liquid All standard state, 25 °c and 1 bar (written to 1 decimal place). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm. Standard Enthalpy Of Formation H2O Liquid.
From studyafrikander.z13.web.core.windows.net
Calculate Standard Enthalpy Of Reaction Standard Enthalpy Of Formation H2O Liquid This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. All standard state, 25. Standard Enthalpy Of Formation H2O Liquid.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation H2O Liquid The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon. Standard Enthalpy Of Formation H2O Liquid.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation H2O Liquid Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of pressure and 298.15.. Standard Enthalpy Of Formation H2O Liquid.
From www.numerade.com
Consider the following reaction 2 H2S (g) + 3 O2 (g) → 2 SO2 (g) + 2 Standard Enthalpy Of Formation H2O Liquid Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of pressure and 298.15. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. Standard Enthalpy Of Formation H2O Liquid.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation H2O Liquid This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of pressure and 298.15.. Standard Enthalpy Of Formation H2O Liquid.
From www.toppr.com
Use the given standard enthalpies of formation (in kJ/mol) to determine Standard Enthalpy Of Formation H2O Liquid The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of pressure and 298.15. Mallard, eds, nist chemistry webbook, nist standard reference database. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. Standard Enthalpy Of Formation H2O Liquid.
From www.nagwa.com
Question Video Calculating the Standard Heat of Reaction for the Standard Enthalpy Of Formation H2O Liquid The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon. Standard Enthalpy Of Formation H2O Liquid.
From joizhadcr.blob.core.windows.net
Standard Enthalpy Of Formation Hcl Aq at Denise Lewis blog Standard Enthalpy Of Formation H2O Liquid 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of pressure and 298.15.. Standard Enthalpy Of Formation H2O Liquid.
From www.chegg.com
Solved Use a standard enthalpies of formation table to Standard Enthalpy Of Formation H2O Liquid Mallard, eds, nist chemistry webbook, nist standard reference database. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide. Standard Enthalpy Of Formation H2O Liquid.
From pafjpegeuw.blogspot.com
How To Calculate Standard Enthalpy Change Using Heats Of Formation Standard Enthalpy Of Formation H2O Liquid 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. Enthalpy of formation (δhf). Standard Enthalpy Of Formation H2O Liquid.
From www.chegg.com
Solved 3) Given the following standard enthalpy change, use Standard Enthalpy Of Formation H2O Liquid 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. All standard state, 25 °c and 1 bar (written to 1 decimal place). Mallard,. Standard Enthalpy Of Formation H2O Liquid.
From chem.libretexts.org
5.7 Enthalpies of Formation Chemistry LibreTexts Standard Enthalpy Of Formation H2O Liquid Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of pressure and 298.15. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the.. Standard Enthalpy Of Formation H2O Liquid.
From rayb78.github.io
Heat Of Formation Chart Standard Enthalpy Of Formation H2O Liquid Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. All standard state, 25 °c and 1 bar (written to 1 decimal place). 193 rows in chemistry and. Standard Enthalpy Of Formation H2O Liquid.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation H2O Liquid All standard state, 25 °c and 1 bar (written to 1 decimal place). Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of formation is. Standard Enthalpy Of Formation H2O Liquid.
From www.toppr.com
The enthalpy of formation of H2O(l) is 280.70 kJ/mol and enthalpy of Standard Enthalpy Of Formation H2O Liquid The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of pressure and 298.15. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The standard enthalpy of formation, also known as the. Standard Enthalpy Of Formation H2O Liquid.
From mavink.com
Nitrogen Enthalpy Chart Standard Enthalpy Of Formation H2O Liquid This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The standard enthalpy of formation,. Standard Enthalpy Of Formation H2O Liquid.
From www.chegg.com
Solved Using the standard enthalpies of formation listed in Standard Enthalpy Of Formation H2O Liquid The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of pressure and 298.15. Mallard, eds, nist chemistry webbook, nist standard reference database. All standard state, 25 °c and 1 bar (written to 1 decimal place). Enthalpy of formation (δhf) is the enthalpy change for the. Standard Enthalpy Of Formation H2O Liquid.
From www.tessshebaylo.com
Balance The Following Chemical Equation And Calculate Standard Standard Enthalpy Of Formation H2O Liquid The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of pressure and 298.15. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen.. Standard Enthalpy Of Formation H2O Liquid.
From www.chegg.com
Solved 13. The standard enthalpies of formation for several Standard Enthalpy Of Formation H2O Liquid 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. This table lists the standard. Standard Enthalpy Of Formation H2O Liquid.