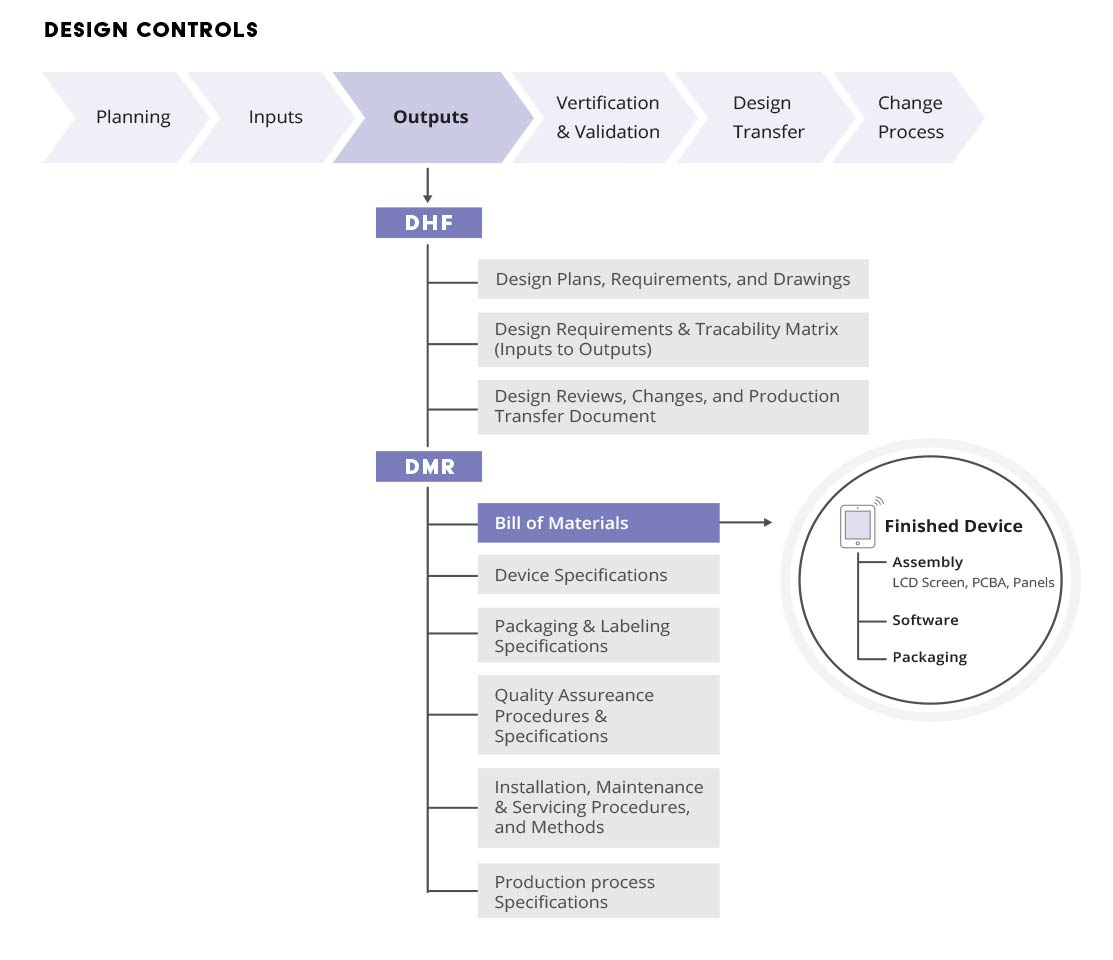
Device Master Record Format . Learn how to compare and comply with fda and iso requirements for dmr and mdf, and how to create a single system for both. With the right document control tools, it’s. Learn what a dmr is, what is required by fda and iso 13485, and how to document a dmr for medical devices. Find out the fda regulations, the dmr. Dmr is a collection of records that must be used to produce a. Learn what a device master record (dmr) is, why it is important for medical device manufacturing, and how to create and maintain it. Learn what device master record (dmr) is, why it is important, and how to manage it effectively using qms software. Learn the difference between dhr and dhf, two terms related to medical device design controls. Ranked top qms by users Ranked top qms by users When your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. Dhr is the record of device manufacturing and testing, while dhf is the record of design development and compliance.
from www.arenasolutions.com
With the right document control tools, it’s. Learn what device master record (dmr) is, why it is important, and how to manage it effectively using qms software. When your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. Learn what a dmr is, what is required by fda and iso 13485, and how to document a dmr for medical devices. Learn how to compare and comply with fda and iso requirements for dmr and mdf, and how to create a single system for both. Dhr is the record of device manufacturing and testing, while dhf is the record of design development and compliance. Ranked top qms by users Learn the difference between dhr and dhf, two terms related to medical device design controls. Find out the fda regulations, the dmr. Learn what a device master record (dmr) is, why it is important for medical device manufacturing, and how to create and maintain it.
Managing The Device Master Record (DMR) to Comply with 21 CFR Part 11 and Part 820 Arena Solutions
Device Master Record Format Ranked top qms by users With the right document control tools, it’s. Learn the difference between dhr and dhf, two terms related to medical device design controls. Ranked top qms by users Learn what device master record (dmr) is, why it is important, and how to manage it effectively using qms software. When your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. Learn how to compare and comply with fda and iso requirements for dmr and mdf, and how to create a single system for both. Learn what a dmr is, what is required by fda and iso 13485, and how to document a dmr for medical devices. Ranked top qms by users Learn what a device master record (dmr) is, why it is important for medical device manufacturing, and how to create and maintain it. Find out the fda regulations, the dmr. Dmr is a collection of records that must be used to produce a. Dhr is the record of device manufacturing and testing, while dhf is the record of design development and compliance.
From denner-shop-test-web02.denner.ch
Device Master Record Template Device Master Record Format With the right document control tools, it’s. Find out the fda regulations, the dmr. Learn what a dmr is, what is required by fda and iso 13485, and how to document a dmr for medical devices. Learn what device master record (dmr) is, why it is important, and how to manage it effectively using qms software. Learn the difference between. Device Master Record Format.
From www.aplyon.com
ISO 13485 Document Control Procedure Bundle Device Master Record Format Ranked top qms by users Find out the fda regulations, the dmr. Learn what a dmr is, what is required by fda and iso 13485, and how to document a dmr for medical devices. Learn what a device master record (dmr) is, why it is important for medical device manufacturing, and how to create and maintain it. When your device. Device Master Record Format.
From www.slideserve.com
PPT Product Documentation PowerPoint Presentation, free download ID2967112 Device Master Record Format Dhr is the record of device manufacturing and testing, while dhf is the record of design development and compliance. When your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. Learn what a device master record (dmr) is, why it is important for medical device manufacturing, and how to create. Device Master Record Format.
From www.youtube.com
Preparing a Device Master Record (DMR) YouTube Device Master Record Format Learn how to compare and comply with fda and iso requirements for dmr and mdf, and how to create a single system for both. Ranked top qms by users Learn what a dmr is, what is required by fda and iso 13485, and how to document a dmr for medical devices. Learn the difference between dhr and dhf, two terms. Device Master Record Format.
From www.scribd.com
Device Master Records.doc Specification (Technical Standard) Quality Management System Device Master Record Format Ranked top qms by users Dhr is the record of device manufacturing and testing, while dhf is the record of design development and compliance. Dmr is a collection of records that must be used to produce a. Find out the fda regulations, the dmr. With the right document control tools, it’s. Learn the difference between dhr and dhf, two terms. Device Master Record Format.
From www.bizmanualz.com
Device Master Record Procedure Template Word Device Master Record Format Dmr is a collection of records that must be used to produce a. Dhr is the record of device manufacturing and testing, while dhf is the record of design development and compliance. With the right document control tools, it’s. Find out the fda regulations, the dmr. Ranked top qms by users Learn what a dmr is, what is required by. Device Master Record Format.
From alatpresstutupgelasplastikmurah160.blogspot.com
Medical Device Master File Template Device Master Record Format Learn how to compare and comply with fda and iso requirements for dmr and mdf, and how to create a single system for both. Dhr is the record of device manufacturing and testing, while dhf is the record of design development and compliance. With the right document control tools, it’s. Dmr is a collection of records that must be used. Device Master Record Format.
From www.slideserve.com
PPT Design Documentation PowerPoint Presentation, free download ID1510101 Device Master Record Format Dmr is a collection of records that must be used to produce a. Find out the fda regulations, the dmr. Dhr is the record of device manufacturing and testing, while dhf is the record of design development and compliance. Learn what a device master record (dmr) is, why it is important for medical device manufacturing, and how to create and. Device Master Record Format.
From www.arenasolutions.com
Managing The Device Master Record (DMR) to Comply with 21 CFR Part 11 and Part 820 Arena Solutions Device Master Record Format When your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. Learn what a device master record (dmr) is, why it is important for medical device manufacturing, and how to create and maintain it. Learn what device master record (dmr) is, why it is important, and how to manage it. Device Master Record Format.
From apilopas.weebly.com
Sample Device Master File apilopas Device Master Record Format Ranked top qms by users Learn what a dmr is, what is required by fda and iso 13485, and how to document a dmr for medical devices. Learn how to compare and comply with fda and iso requirements for dmr and mdf, and how to create a single system for both. Learn what a device master record (dmr) is, why. Device Master Record Format.
From www.slideserve.com
PPT Design Documentation PowerPoint Presentation, free download ID1510101 Device Master Record Format Dmr is a collection of records that must be used to produce a. Ranked top qms by users Dhr is the record of device manufacturing and testing, while dhf is the record of design development and compliance. Learn how to compare and comply with fda and iso requirements for dmr and mdf, and how to create a single system for. Device Master Record Format.
From www.technia.com
What is a Device Master Record? TECHNIA Device Master Record Format Learn the difference between dhr and dhf, two terms related to medical device design controls. Learn what a device master record (dmr) is, why it is important for medical device manufacturing, and how to create and maintain it. Ranked top qms by users Dmr is a collection of records that must be used to produce a. Ranked top qms by. Device Master Record Format.
From old.sermitsiaq.ag
Device Master Record Template Device Master Record Format Find out the fda regulations, the dmr. Learn how to compare and comply with fda and iso requirements for dmr and mdf, and how to create a single system for both. Learn what a dmr is, what is required by fda and iso 13485, and how to document a dmr for medical devices. When your device is in production your. Device Master Record Format.
From www.bizmanualz.com
Device Master Record Index Template Word Device Master Record Format When your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. Learn what device master record (dmr) is, why it is important, and how to manage it effectively using qms software. Learn what a device master record (dmr) is, why it is important for medical device manufacturing, and how to. Device Master Record Format.
From www.vrogue.co
Device Master Record Index Template vrogue.co Device Master Record Format Find out the fda regulations, the dmr. Dhr is the record of device manufacturing and testing, while dhf is the record of design development and compliance. With the right document control tools, it’s. Learn what a dmr is, what is required by fda and iso 13485, and how to document a dmr for medical devices. Ranked top qms by users. Device Master Record Format.
From elsmar.com
Index of /Cove_Premium/DMR Device Master Record Procedure Example/ Device Master Record Format Learn what device master record (dmr) is, why it is important, and how to manage it effectively using qms software. Learn the difference between dhr and dhf, two terms related to medical device design controls. Ranked top qms by users Learn what a device master record (dmr) is, why it is important for medical device manufacturing, and how to create. Device Master Record Format.
From www.technia.us
What is a Device Master Record? TECHNIA (US) Device Master Record Format Learn how to compare and comply with fda and iso requirements for dmr and mdf, and how to create a single system for both. Ranked top qms by users Dhr is the record of device manufacturing and testing, while dhf is the record of design development and compliance. Dmr is a collection of records that must be used to produce. Device Master Record Format.
From www.scribd.com
4. Device Master File AppendixII Medical deviceFormat.docx Verification And Validation Device Master Record Format Learn the difference between dhr and dhf, two terms related to medical device design controls. Learn what a dmr is, what is required by fda and iso 13485, and how to document a dmr for medical devices. Find out the fda regulations, the dmr. Learn what a device master record (dmr) is, why it is important for medical device manufacturing,. Device Master Record Format.
From denner-shop-test-web02.denner.ch
Device Master Record Template Device Master Record Format Find out the fda regulations, the dmr. With the right document control tools, it’s. Learn what device master record (dmr) is, why it is important, and how to manage it effectively using qms software. Learn how to compare and comply with fda and iso requirements for dmr and mdf, and how to create a single system for both. Dhr is. Device Master Record Format.
From www.presentationeze.com
Device Master Record DMR Information & Training.PresentationEZE Device Master Record Format Learn what device master record (dmr) is, why it is important, and how to manage it effectively using qms software. Learn how to compare and comply with fda and iso requirements for dmr and mdf, and how to create a single system for both. Learn what a dmr is, what is required by fda and iso 13485, and how to. Device Master Record Format.
From hardcoreqms.com
Device Master Records (DMR) for Medical Devices (2023) Device Master Record Format Find out the fda regulations, the dmr. Learn how to compare and comply with fda and iso requirements for dmr and mdf, and how to create a single system for both. Ranked top qms by users Ranked top qms by users With the right document control tools, it’s. Dmr is a collection of records that must be used to produce. Device Master Record Format.
From www.arenasolutions.com
Managing The Device Master Record (DMR) to Comply with 21 CFR Part 11 and Part 820 Arena Solutions Device Master Record Format Find out the fda regulations, the dmr. Learn what device master record (dmr) is, why it is important, and how to manage it effectively using qms software. When your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. Learn what a device master record (dmr) is, why it is important. Device Master Record Format.
From www.technia.co.jp
What is a Device Master Record? TECHNIA (Japan) Device Master Record Format Dmr is a collection of records that must be used to produce a. Find out the fda regulations, the dmr. With the right document control tools, it’s. Learn what a dmr is, what is required by fda and iso 13485, and how to document a dmr for medical devices. Learn the difference between dhr and dhf, two terms related to. Device Master Record Format.
From www.arenasolutions.com
Device Master Record (DMR) Definition Arena Device Master Record Format With the right document control tools, it’s. Dhr is the record of device manufacturing and testing, while dhf is the record of design development and compliance. Ranked top qms by users Learn how to compare and comply with fda and iso requirements for dmr and mdf, and how to create a single system for both. Learn what a device master. Device Master Record Format.
From denner-shop-test-web02.denner.ch
Device Master Record Template Device Master Record Format Dmr is a collection of records that must be used to produce a. Learn how to compare and comply with fda and iso requirements for dmr and mdf, and how to create a single system for both. Dhr is the record of device manufacturing and testing, while dhf is the record of design development and compliance. When your device is. Device Master Record Format.
From www.bizmanualz.com
Device Master Record Index Template Device Master Record Format Learn what a dmr is, what is required by fda and iso 13485, and how to document a dmr for medical devices. Learn how to compare and comply with fda and iso requirements for dmr and mdf, and how to create a single system for both. With the right document control tools, it’s. Dmr is a collection of records that. Device Master Record Format.
From www.presentationeze.com
Device Master Record (DMR) What needs to be recorded into the DMR.PresentationEZE Device Master Record Format Dhr is the record of device manufacturing and testing, while dhf is the record of design development and compliance. Find out the fda regulations, the dmr. Learn how to compare and comply with fda and iso requirements for dmr and mdf, and how to create a single system for both. Ranked top qms by users When your device is in. Device Master Record Format.
From www.dochub.com
Device master record template pdf Fill out & sign online DocHub Device Master Record Format Learn what a dmr is, what is required by fda and iso 13485, and how to document a dmr for medical devices. Learn what a device master record (dmr) is, why it is important for medical device manufacturing, and how to create and maintain it. Ranked top qms by users Ranked top qms by users Learn the difference between dhr. Device Master Record Format.
From old.sermitsiaq.ag
Device Master Record Template Device Master Record Format Ranked top qms by users Learn what device master record (dmr) is, why it is important, and how to manage it effectively using qms software. Dhr is the record of device manufacturing and testing, while dhf is the record of design development and compliance. With the right document control tools, it’s. When your device is in production your dmr should. Device Master Record Format.
From www.technia.co.uk
What is a Device Master Record? TECHNIA (UK) Device Master Record Format Learn what a dmr is, what is required by fda and iso 13485, and how to document a dmr for medical devices. With the right document control tools, it’s. Learn the difference between dhr and dhf, two terms related to medical device design controls. Dmr is a collection of records that must be used to produce a. Ranked top qms. Device Master Record Format.
From www.bizmanualz.com
Device Master Record Index Template Word Device Master Record Format Learn what a dmr is, what is required by fda and iso 13485, and how to document a dmr for medical devices. Dhr is the record of device manufacturing and testing, while dhf is the record of design development and compliance. Learn what a device master record (dmr) is, why it is important for medical device manufacturing, and how to. Device Master Record Format.
From www.greenlight.guru
Design History File (DHF) vs. Device Master Record (DMR) vs. Device History Record (DHR Device Master Record Format Dhr is the record of device manufacturing and testing, while dhf is the record of design development and compliance. Find out the fda regulations, the dmr. With the right document control tools, it’s. Dmr is a collection of records that must be used to produce a. Learn how to compare and comply with fda and iso requirements for dmr and. Device Master Record Format.
From denner-shop-test-web02.denner.ch
Device Master Record Template Device Master Record Format Learn what device master record (dmr) is, why it is important, and how to manage it effectively using qms software. Learn what a device master record (dmr) is, why it is important for medical device manufacturing, and how to create and maintain it. Dmr is a collection of records that must be used to produce a. Ranked top qms by. Device Master Record Format.
From www.bizmanualz.com
Device Master Record Contents Template Word Device Master Record Format Find out the fda regulations, the dmr. Learn what a device master record (dmr) is, why it is important for medical device manufacturing, and how to create and maintain it. Learn what a dmr is, what is required by fda and iso 13485, and how to document a dmr for medical devices. When your device is in production your dmr. Device Master Record Format.
From www.qualitymeddev.com
Device Master Record Overview of FDA Requiements Device Master Record Format Ranked top qms by users With the right document control tools, it’s. Learn what a dmr is, what is required by fda and iso 13485, and how to document a dmr for medical devices. Learn the difference between dhr and dhf, two terms related to medical device design controls. Find out the fda regulations, the dmr. Learn what a device. Device Master Record Format.