Standard Enthalpy Of Formation In Methane . the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its. manion, j.a., evaluated enthalpies of formation of the stable closed shell c1 and c2 chlorinated hydrocarbons, j. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. for any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state.
from www.chegg.com
the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. manion, j.a., evaluated enthalpies of formation of the stable closed shell c1 and c2 chlorinated hydrocarbons, j. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. for any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state.
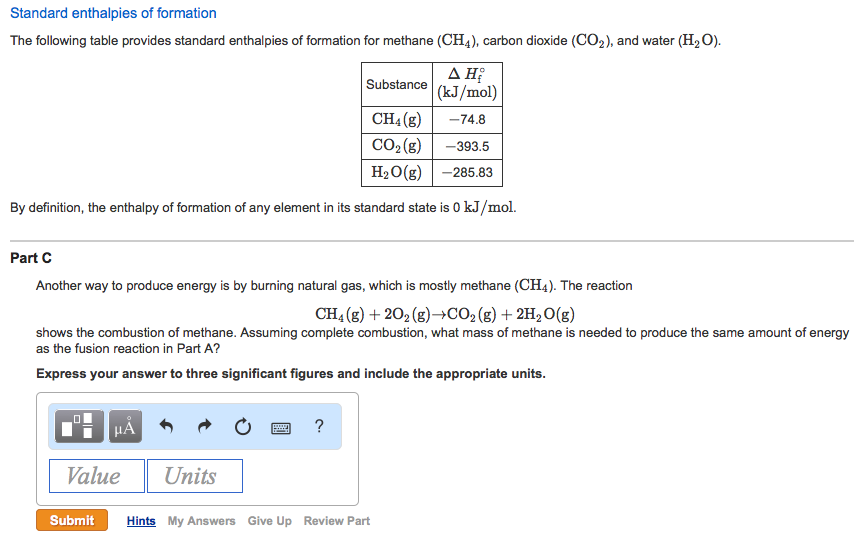
Solved The following table provides standard enthalpies of
Standard Enthalpy Of Formation In Methane 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its. manion, j.a., evaluated enthalpies of formation of the stable closed shell c1 and c2 chlorinated hydrocarbons, j. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. for any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation In Methane definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. for any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the. Standard Enthalpy Of Formation In Methane.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation In Methane manion, j.a., evaluated enthalpies of formation of the stable closed shell c1 and c2 chlorinated hydrocarbons, j. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for.. Standard Enthalpy Of Formation In Methane.
From www.chegg.com
Solved 19. Use the given information to calculate the Standard Enthalpy Of Formation In Methane the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its. definition and explanation of the terms standard state and standard. Standard Enthalpy Of Formation In Methane.
From socratic.org
Given the following data, what is the heat of formation of methane (CH4 Standard Enthalpy Of Formation In Methane manion, j.a., evaluated enthalpies of formation of the stable closed shell c1 and c2 chlorinated hydrocarbons, j. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state.. Standard Enthalpy Of Formation In Methane.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation In Methane manion, j.a., evaluated enthalpies of formation of the stable closed shell c1 and c2 chlorinated hydrocarbons, j. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. for any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a. the standard. Standard Enthalpy Of Formation In Methane.
From www.youtube.com
The enthalpy of combustion of methane, graphite and dihydrogen at 298K Standard Enthalpy Of Formation In Methane for any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its. manion, j.a., evaluated enthalpies of formation of the stable closed shell c1. Standard Enthalpy Of Formation In Methane.
From www.science-revision.co.uk
Enthalpy changes and bond enthalpies Standard Enthalpy Of Formation In Methane the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its. for any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a. definition and explanation of the terms standard state and standard enthalpy of. Standard Enthalpy Of Formation In Methane.
From exoyndeil.blob.core.windows.net
Standard Enthalpy Of Formation In Elements at Michael Zapien blog Standard Enthalpy Of Formation In Methane the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its. manion, j.a., evaluated enthalpies of formation of the stable closed shell c1 and c2 chlorinated hydrocarbons, j. definition and explanation of the terms standard state and standard enthalpy of formation, with listing. Standard Enthalpy Of Formation In Methane.
From surfguppy.com
Calculate the enthalpy change of methane formation using Hess Law Standard Enthalpy Of Formation In Methane for any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its. manion, j.a., evaluated enthalpies of formation of the stable closed shell c1. Standard Enthalpy Of Formation In Methane.
From askfilo.com
The standard enthalpy of formation (ΔfH∘) at 298 K for methane, CH4 ( g),.. Standard Enthalpy Of Formation In Methane the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its. manion, j.a., evaluated enthalpies of formation of the stable closed. Standard Enthalpy Of Formation In Methane.
From www.chegg.com
Solved Determine the enthalpy of combustion of methane (CH4) Standard Enthalpy Of Formation In Methane manion, j.a., evaluated enthalpies of formation of the stable closed shell c1 and c2 chlorinated hydrocarbons, j. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. for any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change. Standard Enthalpy Of Formation In Methane.
From thepresentation.ru
What is enthalpy? презентация, доклад Standard Enthalpy Of Formation In Methane for any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a. manion, j.a., evaluated enthalpies of formation of the stable closed shell c1 and c2 chlorinated hydrocarbons, j. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard. Standard Enthalpy Of Formation In Methane.
From www.numerade.com
SOLVED 3 Calculate the enthalpy of formation of methane, CH4, using Standard Enthalpy Of Formation In Methane 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. for any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. the. Standard Enthalpy Of Formation In Methane.
From www.nagwa.com
Question Video Calculating the Standard Heat of Reaction for the Standard Enthalpy Of Formation In Methane for any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a. manion, j.a., evaluated enthalpies of formation of the stable closed shell c1 and c2 chlorinated hydrocarbons, j. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. the. Standard Enthalpy Of Formation In Methane.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation In Methane definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. for any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a. manion, j.a., evaluated enthalpies of formation of the stable closed shell c1 and c2 chlorinated hydrocarbons, j. 193. Standard Enthalpy Of Formation In Methane.
From byjus.com
under the same conditions heat of formation of water and CO2 are 285 Standard Enthalpy Of Formation In Methane the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. manion, j.a., evaluated enthalpies of formation of the stable closed shell c1 and c2 chlorinated hydrocarbons, j. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation. Standard Enthalpy Of Formation In Methane.
From www.slideserve.com
PPT ENTHALPY OF FORMATION Combustion of Methanol PowerPoint Standard Enthalpy Of Formation In Methane manion, j.a., evaluated enthalpies of formation of the stable closed shell c1 and c2 chlorinated hydrocarbons, j. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. for any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change. Standard Enthalpy Of Formation In Methane.
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube Standard Enthalpy Of Formation In Methane definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each. Standard Enthalpy Of Formation In Methane.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation In Methane the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its. for any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or. Standard Enthalpy Of Formation In Methane.
From www.toppr.com
3. The standard enthalpies of formation of CH4, CO2 and H20 are 74.85 Standard Enthalpy Of Formation In Methane 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state.. Standard Enthalpy Of Formation In Methane.
From www.youtube.com
Hess's law to work out standard enthalpy of formation of methane. YouTube Standard Enthalpy Of Formation In Methane the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. definition and explanation of the terms standard state and standard. Standard Enthalpy Of Formation In Methane.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation In Methane 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. for any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a. manion, j.a., evaluated enthalpies of formation of the stable closed shell c1 and c2 chlorinated hydrocarbons, j. the standard. Standard Enthalpy Of Formation In Methane.
From surfguppy.com
Calculate the enthalpy change of methane formation using Hess Law Standard Enthalpy Of Formation In Methane manion, j.a., evaluated enthalpies of formation of the stable closed shell c1 and c2 chlorinated hydrocarbons, j. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for.. Standard Enthalpy Of Formation In Methane.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation In Methane the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its. for any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a. manion, j.a., evaluated enthalpies of formation of the stable closed shell c1. Standard Enthalpy Of Formation In Methane.
From svanfitz.blogspot.com
Enthalpy Change Of Combustion PPT ENTHALPY OF FORMATION Combustion Standard Enthalpy Of Formation In Methane the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its. definition and explanation of the terms standard state and standard. Standard Enthalpy Of Formation In Methane.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation In Methane manion, j.a., evaluated enthalpies of formation of the stable closed shell c1 and c2 chlorinated hydrocarbons, j. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of. Standard Enthalpy Of Formation In Methane.
From www.chegg.com
Solved The enthalpy change for the reaction in which methane Standard Enthalpy Of Formation In Methane the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its. manion, j.a., evaluated enthalpies of formation of the stable closed shell c1 and c2 chlorinated hydrocarbons, j. for any substance at any particular temperature, we define the standard enthalpy of formation as. Standard Enthalpy Of Formation In Methane.
From askfilo.com
Calculate the enthalpy of formation of methane? Given C(s)+O2 ( g) CO2 Standard Enthalpy Of Formation In Methane manion, j.a., evaluated enthalpies of formation of the stable closed shell c1 and c2 chlorinated hydrocarbons, j. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. for any substance at. Standard Enthalpy Of Formation In Methane.
From www.chegg.com
Solved The following table provides standard enthalpies of Standard Enthalpy Of Formation In Methane for any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a. manion, j.a., evaluated enthalpies of formation of the stable closed shell c1 and c2 chlorinated hydrocarbons, j. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. 193. Standard Enthalpy Of Formation In Methane.
From schoolworkhelper.net
Standard Enthalpies of Formation Online Homework Help SchoolWorkHelper Standard Enthalpy Of Formation In Methane the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. the standard enthalpy of formation is defined as the change in enthalpy when one. Standard Enthalpy Of Formation In Methane.
From www.numerade.com
SOLVEDUse enthalpies of formation below to calculate the standard Standard Enthalpy Of Formation In Methane 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each. Standard Enthalpy Of Formation In Methane.
From www.meritnation.com
Calculate the enthalpy of formation of methane from the following data Standard Enthalpy Of Formation In Methane the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. manion, j.a., evaluated enthalpies of formation of the stable closed shell c1 and c2 chlorinated hydrocarbons, j.. Standard Enthalpy Of Formation In Methane.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID1832797 Standard Enthalpy Of Formation In Methane the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its. manion, j.a., evaluated enthalpies of formation of the stable closed shell c1 and c2 chlorinated hydrocarbons, j. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. Standard Enthalpy Of Formation In Methane.
From www.numerade.com
SOLVED The standard enthalpies of formation, at 25.00 oC, of methane Standard Enthalpy Of Formation In Methane manion, j.a., evaluated enthalpies of formation of the stable closed shell c1 and c2 chlorinated hydrocarbons, j. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. for any substance at. Standard Enthalpy Of Formation In Methane.
From chempedia.info
Enthalpy diagram methane Big Chemical Encyclopedia Standard Enthalpy Of Formation In Methane manion, j.a., evaluated enthalpies of formation of the stable closed shell c1 and c2 chlorinated hydrocarbons, j. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its. the standard enthalpy of formation is defined as the change in enthalpy when one mole. Standard Enthalpy Of Formation In Methane.