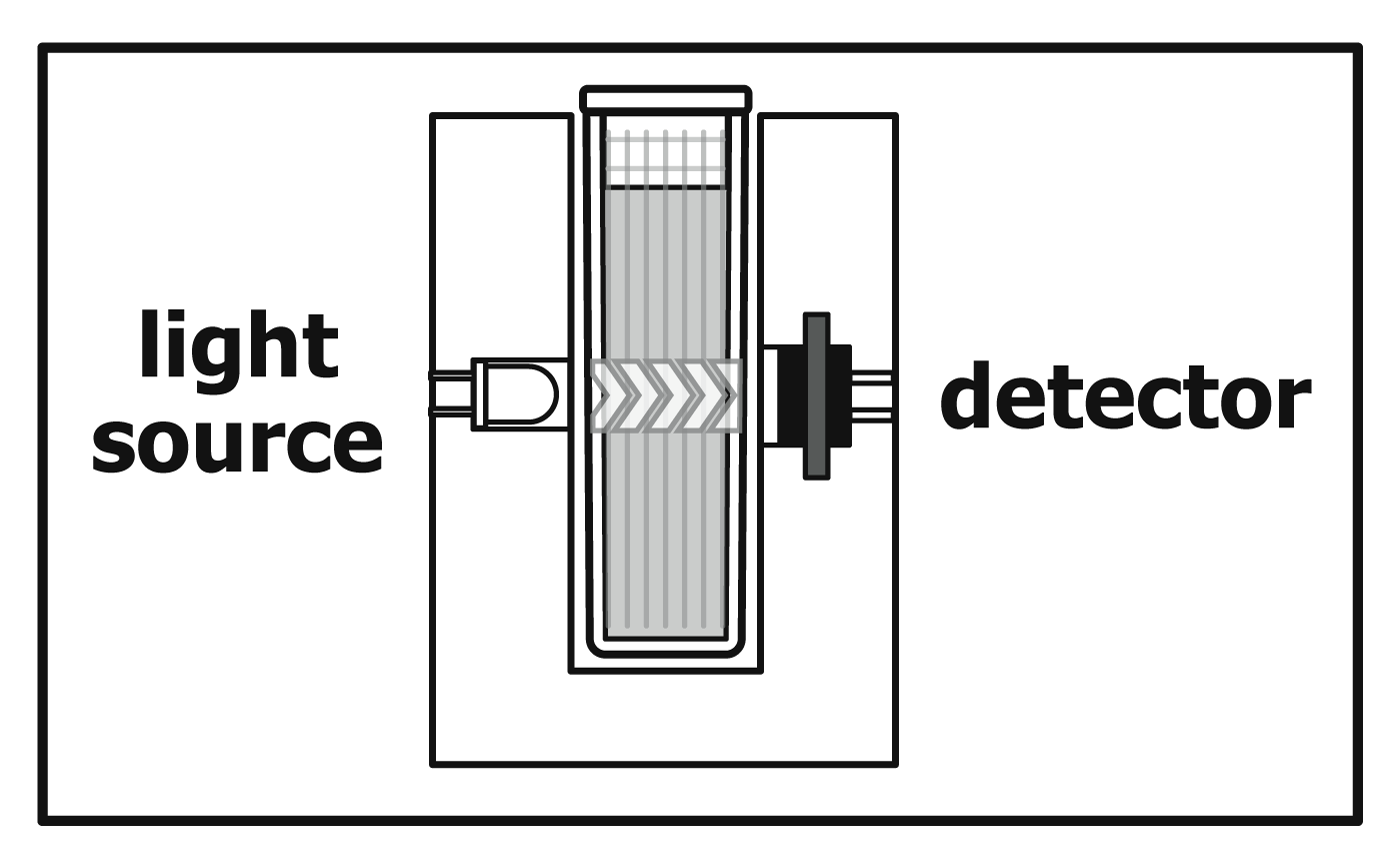
Beer's Law Chemistry . In this section we derive beer's law and consider. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The attenuation of light occurs as a result of distance through solution or increasing concentration. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The premise is that a light beam becomes weaker as it passes through a chemical solution. Beer's law connects absorbance to the concentration of the absorbing species. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance.
from www.vernier.com
In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. In this section we derive beer's law and consider. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The premise is that a light beam becomes weaker as it passes through a chemical solution. The attenuation of light occurs as a result of distance through solution or increasing concentration. Beer's law connects absorbance to the concentration of the absorbing species. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance.
Beer's Law Investigations > Experiment 11 from Investigating Chemistry
Beer's Law Chemistry The attenuation of light occurs as a result of distance through solution or increasing concentration. The attenuation of light occurs as a result of distance through solution or increasing concentration. The premise is that a light beam becomes weaker as it passes through a chemical solution. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer's law connects absorbance to the concentration of the absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. In this section we derive beer's law and consider.
From www.slideserve.com
PPT Determining the Concentration of a Solution Beer’s Law Beer's Law Chemistry In this section we derive beer's law and consider. The premise is that a light beam becomes weaker as it passes through a chemical solution. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The attenuation of light. Beer's Law Chemistry.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Chemistry The premise is that a light beam becomes weaker as it passes through a chemical solution. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer’s law is a relation in spectroscopy that describes the absorption of radiant. Beer's Law Chemistry.
From www.studypool.com
SOLUTION Beer lambert law, definition, history, derivation, diagram Beer's Law Chemistry In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The attenuation of light occurs as a result of distance through solution or increasing concentration. In this section we derive beer's law and consider. The premise is that a light beam becomes weaker as it passes through a chemical solution. Beer's law states that. Beer's Law Chemistry.
From lelandchemclub.weebly.com
Leland Chemistry Club Leland Chemistry Club News Beer's Law Chemistry The premise is that a light beam becomes weaker as it passes through a chemical solution. The attenuation of light occurs as a result of distance through solution or increasing concentration. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In this section we derive beer's law and consider. Beer's law connects absorbance to. Beer's Law Chemistry.
From rrennenextanner.blogspot.com
Beer Lambert Law Ppt rrennenexTanner Beer's Law Chemistry Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The attenuation of light occurs as a result of distance through solution or increasing concentration. In this section we derive beer's law and consider. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. Beer's law connects. Beer's Law Chemistry.
From www.slideserve.com
PPT Beer’s Law and Spectrophotometry PowerPoint Presentation ID211563 Beer's Law Chemistry In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer's law connects absorbance to the concentration of the absorbing species. The premise is that a light beam becomes weaker as it passes through a chemical solution. In this. Beer's Law Chemistry.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Chemistry Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The premise is that a light beam becomes weaker as it. Beer's Law Chemistry.
From hudsontinhoffman.blogspot.com
Beer's Lambert Law Equation HudsontinHoffman Beer's Law Chemistry Beer's law connects absorbance to the concentration of the absorbing species. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption.. Beer's Law Chemistry.
From study.com
How to Find the Absorbance of a Solution Using the BeerLambert Law Beer's Law Chemistry In this section we derive beer's law and consider. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The attenuation of light occurs as a result of distance through solution or increasing concentration. The premise is that a light beam becomes weaker as it passes through a chemical solution. Beer’s law is a relation. Beer's Law Chemistry.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Beer's Law (BeerLambert Law) Beer's Law Chemistry The premise is that a light beam becomes weaker as it passes through a chemical solution. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law connects absorbance to the concentration of the absorbing species. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer’s law. Beer's Law Chemistry.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law Chemistry Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law connects absorbance to the concentration of the absorbing species. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption.. Beer's Law Chemistry.
From www.youtube.com
Spectroscopy Beer Lambert's Law YouTube Beer's Law Chemistry In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The attenuation of light occurs as a result of distance through solution or increasing concentration. The premise is that a light beam becomes weaker as it passes through a chemical solution. In this section we derive beer's law and consider. Beer’s law is a. Beer's Law Chemistry.
From chem.libretexts.org
8.2 Beer's Law Chemistry LibreTexts Beer's Law Chemistry The premise is that a light beam becomes weaker as it passes through a chemical solution. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In this section we derive beer's law and consider. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The attenuation of light. Beer's Law Chemistry.
From www.vernier.com
Beer's Law Investigations > Experiment 11 from Investigating Chemistry Beer's Law Chemistry Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. In this section we derive beer's law and consider. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The attenuation of light occurs as a result of distance through solution or increasing concentration. Beer's law. Beer's Law Chemistry.
From chemistnotes.com
Beer lambert law Derivation, deviation, application, and limitations Beer's Law Chemistry In this section we derive beer's law and consider. The attenuation of light occurs as a result of distance through solution or increasing concentration. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer's law connects absorbance to the concentration of the absorbing species. The premise is that a light beam becomes weaker as. Beer's Law Chemistry.
From chem.libretexts.org
Beer’s Law Chemistry LibreTexts Beer's Law Chemistry The attenuation of light occurs as a result of distance through solution or increasing concentration. In this section we derive beer's law and consider. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. The premise is. Beer's Law Chemistry.
From www.studocu.com
Chem 181 4 Beer's Law Fall 2020 Determining the Concentration of a Beer's Law Chemistry Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In this section we derive beer's law and consider. Beer's law connects absorbance to the concentration of the absorbing species. In most experiments, molar absorptivity (ε) and. Beer's Law Chemistry.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Chemistry The premise is that a light beam becomes weaker as it passes through a chemical solution. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law states that a chemical solution's concentration is directly. Beer's Law Chemistry.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Chemistry Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer's law connects absorbance to the concentration of the absorbing species. The attenuation of light occurs as a result of distance through solution or increasing concentration. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. In. Beer's Law Chemistry.
From www.youtube.com
Spectrophotometry BeerLambert Law. YouTube Beer's Law Chemistry In this section we derive beer's law and consider. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer's law connects absorbance to the concentration of the absorbing species. The premise is that a light beam becomes weaker. Beer's Law Chemistry.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer's Law Chemistry In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law connects absorbance to the concentration of the absorbing species. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. The attenuation of light occurs as a result of distance through solution or increasing concentration.. Beer's Law Chemistry.
From www.youtube.com
Worked example Calculating concentration using the BeerLambert law Beer's Law Chemistry Beer's law connects absorbance to the concentration of the absorbing species. The premise is that a light beam becomes weaker as it passes through a chemical solution. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. In this section we derive beer's law and consider. Beer’s law is a relation in spectroscopy that. Beer's Law Chemistry.
From lelandchemclub.weebly.com
Solutions Leland Chemistry Club Beer's Law Chemistry The premise is that a light beam becomes weaker as it passes through a chemical solution. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. In this section we derive beer's law and consider. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The attenuation. Beer's Law Chemistry.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Beer's Law (BeerLambert Law) Beer's Law Chemistry The attenuation of light occurs as a result of distance through solution or increasing concentration. Beer's law connects absorbance to the concentration of the absorbing species. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. In. Beer's Law Chemistry.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Chemistry Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The premise is that a light beam becomes weaker as it passes through a chemical solution. In this section we derive beer's law and consider. The attenuation of light occurs as a result of distance through solution or increasing concentration. Beer's law connects absorbance to. Beer's Law Chemistry.
From www.youtube.com
Chemical Equilibrium & Beer's Law Intro & Theory YouTube Beer's Law Chemistry In this section we derive beer's law and consider. The attenuation of light occurs as a result of distance through solution or increasing concentration. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In most experiments,. Beer's Law Chemistry.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation Beer's Law Chemistry The attenuation of light occurs as a result of distance through solution or increasing concentration. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In this section we derive beer's law and consider. Beer's law connects absorbance to. Beer's Law Chemistry.
From www.youtube.com
Beer's Law & Spectrophotometry AP Chemistry Complete Course Lesson Beer's Law Chemistry The attenuation of light occurs as a result of distance through solution or increasing concentration. The premise is that a light beam becomes weaker as it passes through a chemical solution. In this section we derive beer's law and consider. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer's law connects absorbance to. Beer's Law Chemistry.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer's Law Chemistry Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The attenuation of light occurs as a result of distance through solution or increasing concentration. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. In this section we derive beer's law and consider. Beer’s law is a relation. Beer's Law Chemistry.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer's Law Chemistry Beer's law connects absorbance to the concentration of the absorbing species. The attenuation of light occurs as a result of distance through solution or increasing concentration. The premise is that a light beam becomes weaker as it passes through a chemical solution. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. In this. Beer's Law Chemistry.
From www.slideserve.com
PPT Exp 14B Determining an Equilibrium Constant PowerPoint Beer's Law Chemistry The attenuation of light occurs as a result of distance through solution or increasing concentration. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. Beer's law connects absorbance to the concentration of the absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is.. Beer's Law Chemistry.
From lukewarmtakes.net
Spectrophotometry and Beer's Law Lukewarm Takes Beer's Law Chemistry Beer's law connects absorbance to the concentration of the absorbing species. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The attenuation of light occurs as a result of distance through solution or increasing concentration.. Beer's Law Chemistry.
From app.formative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Beer's Law Chemistry The attenuation of light occurs as a result of distance through solution or increasing concentration. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. In this section we derive beer's law and consider. Beer's law connects absorbance to. Beer's Law Chemistry.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer's Law Chemistry The premise is that a light beam becomes weaker as it passes through a chemical solution. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In this section we derive beer's law and consider. The attenuation. Beer's Law Chemistry.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law Chemistry The premise is that a light beam becomes weaker as it passes through a chemical solution. Beer's law connects absorbance to the concentration of the absorbing species. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a). Beer's Law Chemistry.