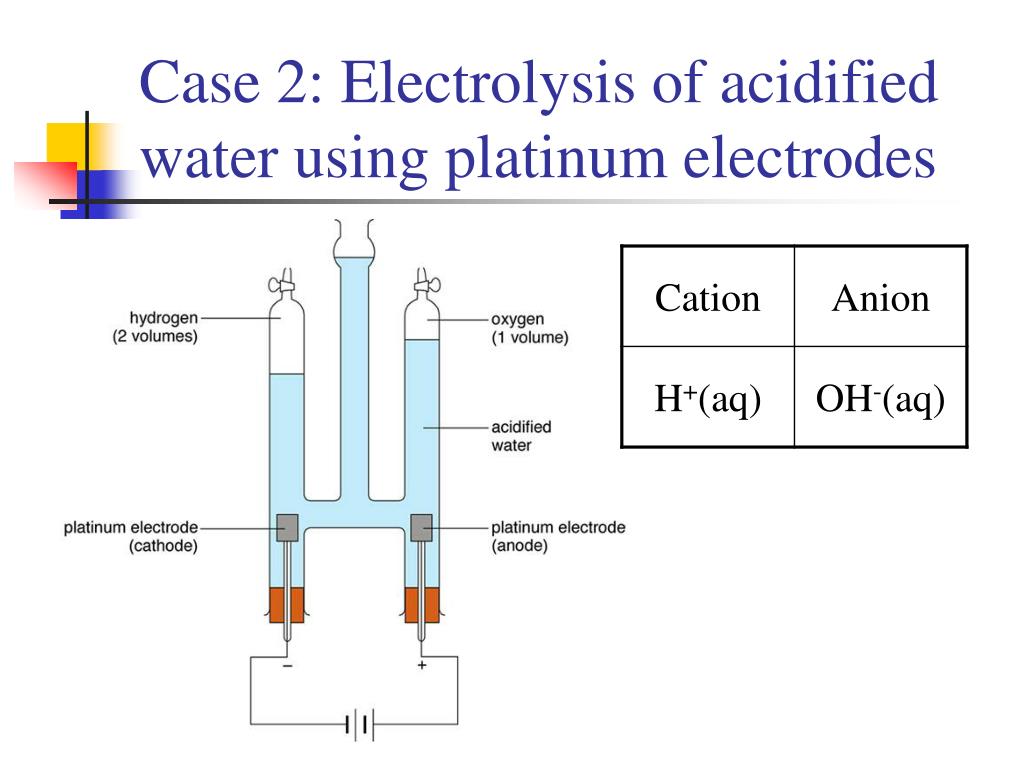
How Electrodes Affect Electrolysis . Two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. The liquid to be electrolyzed must be able to conduct electricity, and so it is usually an aqueous solution of an Ionic compounds conduct electricity when molten or in solution. Electrolysis reactions will not run unless energy is put into the system from outside. An external voltage is applied to drive a nonspontaneous reaction. Reactive metals are extracted from their ores using electrolysis. Here, we use three case studies that illustrate three key concepts associated with electrosynthesis, the impact of the nature of the. In the case of electrolysis reactions, the energy is provided by the battery. Electrolysis is breaking up an ionic compound (either molten or in solution) into simpler substances by using an electric current. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. These electrodes are often made of an inert material such as stainless steel, platinum, or graphite. Electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. The material composition, surface area, and reactivity of the electrodes can significantly influence the rate of reaction, the formation of by. In an electrolytic cell, however, the opposite process, called electrolysis, occurs:
from www.slideserve.com
Electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. Here, we use three case studies that illustrate three key concepts associated with electrosynthesis, the impact of the nature of the. Electrolysis reactions will not run unless energy is put into the system from outside. Electrolysis is breaking up an ionic compound (either molten or in solution) into simpler substances by using an electric current. Two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. The liquid to be electrolyzed must be able to conduct electricity, and so it is usually an aqueous solution of an In the case of electrolysis reactions, the energy is provided by the battery. The material composition, surface area, and reactivity of the electrodes can significantly influence the rate of reaction, the formation of by. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. In an electrolytic cell, however, the opposite process, called electrolysis, occurs:
PPT Electrolysis PowerPoint Presentation, free download ID297961
How Electrodes Affect Electrolysis For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. Here, we use three case studies that illustrate three key concepts associated with electrosynthesis, the impact of the nature of the. Ionic compounds conduct electricity when molten or in solution. Two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. The liquid to be electrolyzed must be able to conduct electricity, and so it is usually an aqueous solution of an Electrolysis is breaking up an ionic compound (either molten or in solution) into simpler substances by using an electric current. The material composition, surface area, and reactivity of the electrodes can significantly influence the rate of reaction, the formation of by. Reactive metals are extracted from their ores using electrolysis. These electrodes are often made of an inert material such as stainless steel, platinum, or graphite. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. Electrolysis reactions will not run unless energy is put into the system from outside. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. In the case of electrolysis reactions, the energy is provided by the battery. An external voltage is applied to drive a nonspontaneous reaction.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts How Electrodes Affect Electrolysis In the case of electrolysis reactions, the energy is provided by the battery. Two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. These electrodes are often made of an inert material such as stainless steel, platinum, or graphite. Here, we use three case studies that illustrate three key concepts associated with electrosynthesis, the impact of the nature. How Electrodes Affect Electrolysis.
From uen.pressbooks.pub
Electrolysis Introductory Chemistry How Electrodes Affect Electrolysis Two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. Electrolysis is breaking up an ionic compound (either molten or in solution) into simpler substances by using an electric current. In the case of electrolysis reactions, the energy is provided by the battery. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Electrolysis reactions will not. How Electrodes Affect Electrolysis.
From exotsdtks.blob.core.windows.net
Electrolysis Electrodes Reaction at Leroy Russell blog How Electrodes Affect Electrolysis These electrodes are often made of an inert material such as stainless steel, platinum, or graphite. Two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. In the case of electrolysis reactions, the energy is provided by the battery. The liquid to be electrolyzed must be able to conduct electricity, and so it is usually an aqueous solution. How Electrodes Affect Electrolysis.
From app.pandai.org
Electrolytic Cell How Electrodes Affect Electrolysis For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. Ionic compounds conduct electricity when molten or in solution. Here, we use three case studies that illustrate three key concepts associated with electrosynthesis, the impact of the nature of the. Electrolysis is breaking up an ionic compound (either molten. How Electrodes Affect Electrolysis.
From chem.libretexts.org
11.7 Electrolysis Chemistry LibreTexts How Electrodes Affect Electrolysis In an electrolytic cell, however, the opposite process, called electrolysis, occurs: The liquid to be electrolyzed must be able to conduct electricity, and so it is usually an aqueous solution of an Two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to. How Electrodes Affect Electrolysis.
From www.youtube.com
Electrolysis of Water Electrochemistry YouTube How Electrodes Affect Electrolysis Two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. Ionic compounds conduct electricity when molten or in solution. The liquid to be electrolyzed must be able to conduct electricity, and so it is usually an aqueous solution of an In an electrolytic cell, however, the opposite process, called electrolysis, occurs: The material composition, surface area, and reactivity. How Electrodes Affect Electrolysis.
From www.adevos.science
Electrolysis Adevoscience How Electrodes Affect Electrolysis The material composition, surface area, and reactivity of the electrodes can significantly influence the rate of reaction, the formation of by. Electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. These electrodes are often made of an inert material such as stainless steel, platinum, or graphite. The liquid to be electrolyzed must be able. How Electrodes Affect Electrolysis.
From www.youtube.com
Electrolysis Preferential discharge 2 (nature of electrode) YouTube How Electrodes Affect Electrolysis Electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. An external voltage is applied to drive a nonspontaneous reaction. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. Here, we use three case studies that illustrate three key concepts associated with electrosynthesis,. How Electrodes Affect Electrolysis.
From fyodeauzx.blob.core.windows.net
Electrode Effect Electrolysis at Edna Boardman blog How Electrodes Affect Electrolysis Electrolysis is breaking up an ionic compound (either molten or in solution) into simpler substances by using an electric current. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. The liquid to be electrolyzed must be able to conduct electricity, and so it is usually an aqueous solution. How Electrodes Affect Electrolysis.
From spmscience.blog.onlinetuition.com.my
Electrolysis SPM Science How Electrodes Affect Electrolysis The material composition, surface area, and reactivity of the electrodes can significantly influence the rate of reaction, the formation of by. These electrodes are often made of an inert material such as stainless steel, platinum, or graphite. Electrolysis reactions will not run unless energy is put into the system from outside. Electrolysis separates chemically bonded ionic substances and compounds by. How Electrodes Affect Electrolysis.
From owlcation.com
Electrolysis The Way of the Future Owlcation How Electrodes Affect Electrolysis For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. In the case of electrolysis reactions, the energy is provided by the battery. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Ionic compounds conduct electricity when molten or in solution. The liquid to be electrolyzed must. How Electrodes Affect Electrolysis.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic How Electrodes Affect Electrolysis The material composition, surface area, and reactivity of the electrodes can significantly influence the rate of reaction, the formation of by. Electrolysis is breaking up an ionic compound (either molten or in solution) into simpler substances by using an electric current. Electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. In an electrolytic cell,. How Electrodes Affect Electrolysis.
From www.researchgate.net
Effect of electrolysis time on (a) electrode consumption (b) energy How Electrodes Affect Electrolysis The material composition, surface area, and reactivity of the electrodes can significantly influence the rate of reaction, the formation of by. Electrolysis is breaking up an ionic compound (either molten or in solution) into simpler substances by using an electric current. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction. How Electrodes Affect Electrolysis.
From electro-lysis.blogspot.com
Electro electricity lysisbreak down How Electrodes Affect Electrolysis An external voltage is applied to drive a nonspontaneous reaction. Two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. Ionic compounds conduct electricity when molten or in solution. The liquid to be electrolyzed must be able to conduct electricity, and so it is usually an aqueous solution of an These electrodes are often made of an inert. How Electrodes Affect Electrolysis.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID6499367 How Electrodes Affect Electrolysis In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Reactive metals are extracted from their ores using electrolysis. These electrodes are often made of an inert material such as stainless steel, platinum, or graphite. Ionic compounds conduct electricity when molten or in solution. The material composition, surface area, and reactivity of the electrodes can significantly influence the rate. How Electrodes Affect Electrolysis.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace How Electrodes Affect Electrolysis The liquid to be electrolyzed must be able to conduct electricity, and so it is usually an aqueous solution of an Reactive metals are extracted from their ores using electrolysis. Electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. An external voltage is applied to drive a nonspontaneous reaction. In the case of electrolysis. How Electrodes Affect Electrolysis.
From mungfali.com
Electrolysis Process Diagram How Electrodes Affect Electrolysis The material composition, surface area, and reactivity of the electrodes can significantly influence the rate of reaction, the formation of by. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. Two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. An external voltage is applied to. How Electrodes Affect Electrolysis.
From icsechemistry16.blogspot.com
Electrolysis of acidified water with Platinum electrodes How Electrodes Affect Electrolysis For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. An external voltage is applied to drive a nonspontaneous reaction. Electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. Ionic compounds conduct electricity when molten or in solution. Electrolysis reactions will not run. How Electrodes Affect Electrolysis.
From www.snexplores.org
Explainer What is an electrode? How Electrodes Affect Electrolysis Here, we use three case studies that illustrate three key concepts associated with electrosynthesis, the impact of the nature of the. Ionic compounds conduct electricity when molten or in solution. Electrolysis reactions will not run unless energy is put into the system from outside. Reactive metals are extracted from their ores using electrolysis. Electrolysis separates chemically bonded ionic substances and. How Electrodes Affect Electrolysis.
From fyodeauzx.blob.core.windows.net
Electrode Effect Electrolysis at Edna Boardman blog How Electrodes Affect Electrolysis Reactive metals are extracted from their ores using electrolysis. Two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. Here, we use three case studies that illustrate three key concepts associated with electrosynthesis, the impact of the nature of the. These electrodes are often made of an inert material such as stainless steel, platinum, or graphite. The material. How Electrodes Affect Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation ID297961 How Electrodes Affect Electrolysis Electrolysis reactions will not run unless energy is put into the system from outside. Here, we use three case studies that illustrate three key concepts associated with electrosynthesis, the impact of the nature of the. Reactive metals are extracted from their ores using electrolysis. The material composition, surface area, and reactivity of the electrodes can significantly influence the rate of. How Electrodes Affect Electrolysis.
From courses.lumenlearning.com
Predicting the Products of Electrolysis Introduction to Chemistry How Electrodes Affect Electrolysis The liquid to be electrolyzed must be able to conduct electricity, and so it is usually an aqueous solution of an Ionic compounds conduct electricity when molten or in solution. An external voltage is applied to drive a nonspontaneous reaction. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to. How Electrodes Affect Electrolysis.
From helpiks.org
ELECTROLYSIS OF AQUEOUSSOLUTIONS How Electrodes Affect Electrolysis An external voltage is applied to drive a nonspontaneous reaction. Two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. In the case of electrolysis reactions, the energy is provided by the battery. Electrolysis reactions will not run unless energy is put into the system from outside. Reactive metals are extracted from their ores using electrolysis. The liquid. How Electrodes Affect Electrolysis.
From study.com
Electrolysis Definition, Reaction & Process Lesson How Electrodes Affect Electrolysis Reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in solution. Electrolysis is breaking up an ionic compound (either molten or in solution) into simpler substances by using an electric current. The liquid to be electrolyzed must be able to conduct electricity, and so it is usually an aqueous solution of an These. How Electrodes Affect Electrolysis.
From www.youtube.com
Electrolysis of Copper Sulphate YouTube How Electrodes Affect Electrolysis Electrolysis reactions will not run unless energy is put into the system from outside. The material composition, surface area, and reactivity of the electrodes can significantly influence the rate of reaction, the formation of by. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. Electrolysis separates chemically bonded. How Electrodes Affect Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 How Electrodes Affect Electrolysis In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Ionic compounds conduct electricity when molten or in solution. The material composition, surface area, and reactivity of the electrodes can significantly influence the rate of reaction, the formation of by. An external voltage is applied to drive a nonspontaneous reaction. Reactive metals are extracted from their ores using electrolysis.. How Electrodes Affect Electrolysis.
From mungfali.com
Water Electrolysis Process How Electrodes Affect Electrolysis Here, we use three case studies that illustrate three key concepts associated with electrosynthesis, the impact of the nature of the. An external voltage is applied to drive a nonspontaneous reaction. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Ionic compounds conduct electricity when molten or in solution. The material composition, surface area, and reactivity of the. How Electrodes Affect Electrolysis.
From www.slideserve.com
PPT CHAPTER 6 ELECTROLYSIS PowerPoint Presentation, free download How Electrodes Affect Electrolysis In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Reactive metals are extracted from their ores using electrolysis. Electrolysis reactions will not run unless energy is put into the system from outside. Here, we use three case studies that illustrate three key concepts associated with electrosynthesis, the impact of the nature of the. These electrodes are often made. How Electrodes Affect Electrolysis.
From stoplearn.com
Electrolysis Notes 2023 How Electrodes Affect Electrolysis The material composition, surface area, and reactivity of the electrodes can significantly influence the rate of reaction, the formation of by. Ionic compounds conduct electricity when molten or in solution. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. Electrolysis reactions will not run unless energy is put. How Electrodes Affect Electrolysis.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download How Electrodes Affect Electrolysis Here, we use three case studies that illustrate three key concepts associated with electrosynthesis, the impact of the nature of the. Electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. An external voltage. How Electrodes Affect Electrolysis.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis How Electrodes Affect Electrolysis Electrolysis is breaking up an ionic compound (either molten or in solution) into simpler substances by using an electric current. Two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. Electrolysis reactions will not run unless energy is put into the system from outside. Reactive metals are extracted from their ores using electrolysis. An external voltage is applied. How Electrodes Affect Electrolysis.
From fyodeauzx.blob.core.windows.net
Electrode Effect Electrolysis at Edna Boardman blog How Electrodes Affect Electrolysis For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. The liquid to be electrolyzed must be able to conduct electricity, and so it is usually an aqueous solution of an An external voltage is applied to drive a nonspontaneous reaction. In an electrolytic cell, however, the opposite process,. How Electrodes Affect Electrolysis.
From www.slideserve.com
PPT Electrolysis L.O. I know and can use the terms electrolyte How Electrodes Affect Electrolysis Ionic compounds conduct electricity when molten or in solution. These electrodes are often made of an inert material such as stainless steel, platinum, or graphite. Electrolysis reactions will not run unless energy is put into the system from outside. An external voltage is applied to drive a nonspontaneous reaction. The liquid to be electrolyzed must be able to conduct electricity,. How Electrodes Affect Electrolysis.
From giowrvapv.blob.core.windows.net
What Electrodes Are Used In Electrolysis at Patricia Nutt blog How Electrodes Affect Electrolysis Ionic compounds conduct electricity when molten or in solution. The material composition, surface area, and reactivity of the electrodes can significantly influence the rate of reaction, the formation of by. Electrolysis is breaking up an ionic compound (either molten or in solution) into simpler substances by using an electric current. An external voltage is applied to drive a nonspontaneous reaction.. How Electrodes Affect Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 How Electrodes Affect Electrolysis These electrodes are often made of an inert material such as stainless steel, platinum, or graphite. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. The material composition, surface area, and reactivity of the electrodes can significantly influence the rate of reaction, the formation of by. The liquid. How Electrodes Affect Electrolysis.