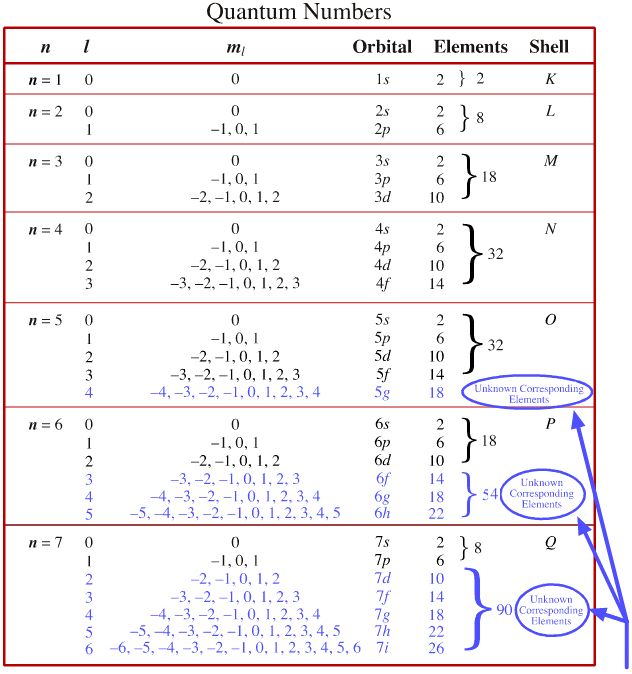
What Are The Set Of Quantum Numbers . They represent the position, movement, and energy of the fundamental particle. There are four quantum numbers: N, ℓ, m ℓ, and m s. The original three quantum numbers describe. Each one is a particular factor in an equation describing a property of the electron. There are four quantum numbers that we are going to discuss: Originally, there were three quantum numbers, the ones needed for the calculation of the key equation of quantum physics, schrodinger’s equation. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. In atoms, there are a total of four quantum numbers: Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides.
from shanimchemistry.blogspot.com
N, ℓ, m ℓ, and m s. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic. Originally, there were three quantum numbers, the ones needed for the calculation of the key equation of quantum physics, schrodinger’s equation. There are four quantum numbers: There are four quantum numbers that we are going to discuss: The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. In atoms, there are a total of four quantum numbers: The original three quantum numbers describe. They represent the position, movement, and energy of the fundamental particle.
*CHEMISTRY MATRICULATION* QUANTUM NUMBERS
What Are The Set Of Quantum Numbers N, ℓ, m ℓ, and m s. Originally, there were three quantum numbers, the ones needed for the calculation of the key equation of quantum physics, schrodinger’s equation. N, ℓ, m ℓ, and m s. They represent the position, movement, and energy of the fundamental particle. The original three quantum numbers describe. There are four quantum numbers that we are going to discuss: In atoms, there are a total of four quantum numbers: The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. Each one is a particular factor in an equation describing a property of the electron. There are four quantum numbers: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic.
From www.youtube.com
Example Writing all sets of 4 Quantum Numbers for the 4d subshell What Are The Set Of Quantum Numbers There are four quantum numbers that we are going to discuss: The original three quantum numbers describe. N, ℓ, m ℓ, and m s. Originally, there were three quantum numbers, the ones needed for the calculation of the key equation of quantum physics, schrodinger’s equation. Quantum numbers are a set of numbers used to define the state in which a. What Are The Set Of Quantum Numbers.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID4952601 What Are The Set Of Quantum Numbers Originally, there were three quantum numbers, the ones needed for the calculation of the key equation of quantum physics, schrodinger’s equation. Each one is a particular factor in an equation describing a property of the electron. There are four quantum numbers that we are going to discuss: The principal quantum number (n), the orbital angular momentum quantum number (l), the. What Are The Set Of Quantum Numbers.
From byjus.com
the correct set of four quantum numbers for the valance electron of kr What Are The Set Of Quantum Numbers In atoms, there are a total of four quantum numbers: Each one is a particular factor in an equation describing a property of the electron. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. Quantum numbers are a set of numbers used to define the state in which a fundamental. What Are The Set Of Quantum Numbers.
From studylib.net
Quantum Numbers What Are The Set Of Quantum Numbers N, ℓ, m ℓ, and m s. The original three quantum numbers describe. Originally, there were three quantum numbers, the ones needed for the calculation of the key equation of quantum physics, schrodinger’s equation. There are four quantum numbers: There are four quantum numbers that we are going to discuss: Quantum numbers are a set of numbers used to define. What Are The Set Of Quantum Numbers.
From www.w3schools.blog
Quantum Numbers W3schools What Are The Set Of Quantum Numbers N, ℓ, m ℓ, and m s. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic. They represent the position, movement, and energy of the fundamental particle. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. Each one is a particular factor in an equation. What Are The Set Of Quantum Numbers.
From www.slideserve.com
PPT Quantum Numbers and Electronic Configuration PowerPoint What Are The Set Of Quantum Numbers Each one is a particular factor in an equation describing a property of the electron. In atoms, there are a total of four quantum numbers: There are four quantum numbers: Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. They represent the position, movement,. What Are The Set Of Quantum Numbers.
From mavink.com
Quantum Numbers Symbols What Are The Set Of Quantum Numbers N, ℓ, m ℓ, and m s. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. There are four quantum numbers that we are going to discuss: Originally, there were three quantum numbers, the ones needed for the calculation of the key equation of quantum physics, schrodinger’s equation. The principal. What Are The Set Of Quantum Numbers.
From www.toppr.com
The set of quantum numbers n = 3, l = 2, ml = 0 What Are The Set Of Quantum Numbers In atoms, there are a total of four quantum numbers: There are four quantum numbers: N, ℓ, m ℓ, and m s. Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. Originally, there were three quantum numbers, the ones needed for the calculation of. What Are The Set Of Quantum Numbers.
From www.priyamstudycentre.com
Quantum Number Orbitals Diagram, Definition, Chart, Shape What Are The Set Of Quantum Numbers Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. There are four quantum numbers that we are going to discuss: The original three quantum numbers describe. There are four quantum numbers: They represent the position, movement, and energy of the fundamental particle. The principal. What Are The Set Of Quantum Numbers.
From www.slideserve.com
PPT Quantum Theory PowerPoint Presentation, free download ID1185278 What Are The Set Of Quantum Numbers Each one is a particular factor in an equation describing a property of the electron. N, ℓ, m ℓ, and m s. There are four quantum numbers: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic. The original three quantum numbers describe. The quantum numbers are parameters that describe the distribution of electrons in the. What Are The Set Of Quantum Numbers.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID6766216 What Are The Set Of Quantum Numbers The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. Each one is a particular factor in an equation describing a property of the electron. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic. There are four quantum numbers that we are going to discuss: N,. What Are The Set Of Quantum Numbers.
From savekhaoyai.blogspot.com
41 quantum number practice worksheet Worksheet Database What Are The Set Of Quantum Numbers They represent the position, movement, and energy of the fundamental particle. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. There are four quantum numbers that we are going to discuss: Each one is a particular. What Are The Set Of Quantum Numbers.
From www.slideserve.com
PPT Electron Configurations & Quantum Numbers PowerPoint Presentation What Are The Set Of Quantum Numbers Each one is a particular factor in an equation describing a property of the electron. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic. In atoms, there are a total of four quantum numbers: N, ℓ,. What Are The Set Of Quantum Numbers.
From shanimchemistry.blogspot.com
*CHEMISTRY MATRICULATION* QUANTUM NUMBERS What Are The Set Of Quantum Numbers There are four quantum numbers that we are going to discuss: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic. Originally, there were three quantum numbers, the ones needed for the calculation of the key equation of quantum physics, schrodinger’s equation. N, ℓ, m ℓ, and m s. Each one is a particular factor in. What Are The Set Of Quantum Numbers.
From www.slideserve.com
PPT Quantum Mechanical Model PowerPoint Presentation, free download What Are The Set Of Quantum Numbers Originally, there were three quantum numbers, the ones needed for the calculation of the key equation of quantum physics, schrodinger’s equation. There are four quantum numbers that we are going to discuss: Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. In atoms, there. What Are The Set Of Quantum Numbers.
From www.slideserve.com
PPT Quantum numbers and orbital energies Each atom’s electron has a What Are The Set Of Quantum Numbers Each one is a particular factor in an equation describing a property of the electron. The original three quantum numbers describe. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic. They represent the position, movement, and. What Are The Set Of Quantum Numbers.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID3181772 What Are The Set Of Quantum Numbers They represent the position, movement, and energy of the fundamental particle. There are four quantum numbers that we are going to discuss: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic. In atoms, there are a total of four quantum numbers: The quantum numbers are parameters that describe the distribution of electrons in the atom,. What Are The Set Of Quantum Numbers.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID3181772 What Are The Set Of Quantum Numbers Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. In atoms, there are a total of four quantum numbers: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic. There are four quantum numbers: There are four quantum numbers that. What Are The Set Of Quantum Numbers.
From www.youtube.com
Quantum Numbers YouTube What Are The Set Of Quantum Numbers In atoms, there are a total of four quantum numbers: Each one is a particular factor in an equation describing a property of the electron. N, ℓ, m ℓ, and m s. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic. Originally, there were three quantum numbers, the ones needed for the calculation of the. What Are The Set Of Quantum Numbers.
From www.slideserve.com
PPT Quantum numbers and orbital energies Each atom’s electron has a What Are The Set Of Quantum Numbers Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. In atoms, there are a total of four quantum numbers: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic. The quantum numbers are parameters that describe the distribution of electrons. What Are The Set Of Quantum Numbers.
From stock.adobe.com
table of quantum numbers Stock Vector Adobe Stock What Are The Set Of Quantum Numbers N, ℓ, m ℓ, and m s. The original three quantum numbers describe. There are four quantum numbers: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic. Originally, there were three quantum numbers, the ones needed for the calculation of the key equation of quantum physics, schrodinger’s equation. Quantum numbers are a set of numbers. What Are The Set Of Quantum Numbers.
From www.youtube.com
Quantum numbers Electronic structure of atoms Chemistry Khan What Are The Set Of Quantum Numbers N, ℓ, m ℓ, and m s. Each one is a particular factor in an equation describing a property of the electron. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. Originally, there were three quantum numbers, the ones needed for the calculation of the key equation of quantum physics,. What Are The Set Of Quantum Numbers.
From www.slideserve.com
PPT Chapter 2 Atomic Structure and Interatomic Bonding PowerPoint What Are The Set Of Quantum Numbers They represent the position, movement, and energy of the fundamental particle. There are four quantum numbers: Originally, there were three quantum numbers, the ones needed for the calculation of the key equation of quantum physics, schrodinger’s equation. Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and. What Are The Set Of Quantum Numbers.
From byjus.com
Quantum Numbers Types of Quantum Numbers Chemistry Byju's What Are The Set Of Quantum Numbers The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic. There are four quantum numbers: The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus,. What Are The Set Of Quantum Numbers.
From eduinput.com
Quantum numbersPrinciple, Azimuthal, and Spin What Are The Set Of Quantum Numbers N, ℓ, m ℓ, and m s. There are four quantum numbers: In atoms, there are a total of four quantum numbers: They represent the position, movement, and energy of the fundamental particle. There are four quantum numbers that we are going to discuss: Originally, there were three quantum numbers, the ones needed for the calculation of the key equation. What Are The Set Of Quantum Numbers.
From study.com
Quantum Numbers on the Periodic Table Definition & Overview Lesson What Are The Set Of Quantum Numbers N, ℓ, m ℓ, and m s. Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. They represent the position, movement, and energy of the fundamental particle. Each one is a particular factor in an equation describing a property of the electron. The original. What Are The Set Of Quantum Numbers.
From www.slideserve.com
PPT Chapter 7 Periodicity and Atomic Structure PowerPoint What Are The Set Of Quantum Numbers N, ℓ, m ℓ, and m s. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. The original three quantum numbers describe. In atoms, there are a total of four quantum numbers: Quantum numbers are a. What Are The Set Of Quantum Numbers.
From en.ppt-online.org
Atomic structure and properties. (Chapter 3) online presentation What Are The Set Of Quantum Numbers The original three quantum numbers describe. They represent the position, movement, and energy of the fundamental particle. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic. Originally, there were three quantum numbers, the ones needed for the calculation of the key equation of quantum physics, schrodinger’s equation. The quantum numbers are parameters that describe the. What Are The Set Of Quantum Numbers.
From quantum-lesson.blogspot.com
How To Set Up Quantum Numbers What Are The Set Of Quantum Numbers N, ℓ, m ℓ, and m s. In atoms, there are a total of four quantum numbers: There are four quantum numbers: Originally, there were three quantum numbers, the ones needed for the calculation of the key equation of quantum physics, schrodinger’s equation. Each one is a particular factor in an equation describing a property of the electron. They represent. What Are The Set Of Quantum Numbers.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition What Are The Set Of Quantum Numbers They represent the position, movement, and energy of the fundamental particle. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic. There are four quantum numbers that we are going to discuss: There are four quantum numbers: The original three quantum numbers describe. Originally, there were three quantum numbers, the ones needed for the calculation of. What Are The Set Of Quantum Numbers.
From chemistrypubs.com
Quantum Numbers Principle, Examples, Significance Chemistrupubs What Are The Set Of Quantum Numbers They represent the position, movement, and energy of the fundamental particle. N, ℓ, m ℓ, and m s. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic. There are four quantum numbers that we are going to discuss: The original three quantum numbers describe. Quantum numbers are a set of numbers used to define the. What Are The Set Of Quantum Numbers.
From fity.club
Quantum Numbers Diagram What Are The Set Of Quantum Numbers The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic. In atoms, there are a total of four quantum numbers: Each one is a particular factor in an equation describing a property of the electron. There are four quantum numbers that we are going to discuss: There are four quantum numbers: The quantum numbers are parameters. What Are The Set Of Quantum Numbers.
From andreaminsharp.blogspot.com
Set of Quantum Numbers AndreaminSharp What Are The Set Of Quantum Numbers There are four quantum numbers that we are going to discuss: Each one is a particular factor in an equation describing a property of the electron. There are four quantum numbers: The original three quantum numbers describe. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic. In atoms, there are a total of four quantum. What Are The Set Of Quantum Numbers.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition What Are The Set Of Quantum Numbers There are four quantum numbers that we are going to discuss: Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. They represent the position, movement, and energy of the fundamental particle. The original three quantum numbers describe. The quantum numbers are parameters that describe. What Are The Set Of Quantum Numbers.
From www.geeksforgeeks.org
Quantum Numbers Principal, Azimuthal, & Spin What Are The Set Of Quantum Numbers They represent the position, movement, and energy of the fundamental particle. There are four quantum numbers: The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. In atoms, there are a total of four quantum numbers: N, ℓ, m ℓ, and m s. Quantum numbers are a set of numbers used. What Are The Set Of Quantum Numbers.