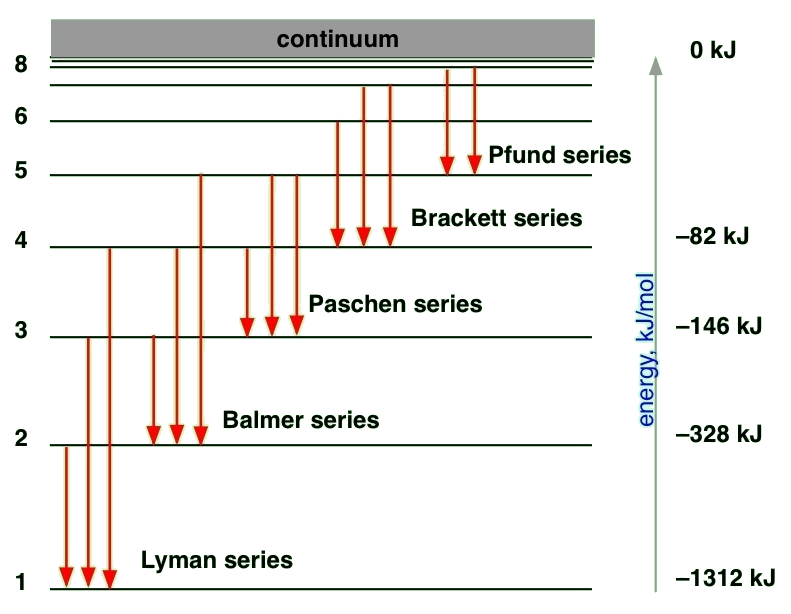
Bohr Model Energy Levels . Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher energy level, then immediately. Describe the triumphs and limits of bohr’s theory. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Niels bohr’s quantum model assumed that the electron could only orbit with certain allowed radii. Explain bohr’s planetary model of the atom. Although the bohr model is regarded as inaccurate and outdated, the model predicts the hydrogen atom well and provides good predictions on quantized energy levels. Each orbit was associated with an energy level. Drawing a shell model diagram and an energy diagram for hydrogen, and then using the. Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher. Calculating electron energy for levels n=1 to 3.
from chem.libretexts.org
Calculating electron energy for levels n=1 to 3. Each orbit was associated with an energy level. Niels bohr’s quantum model assumed that the electron could only orbit with certain allowed radii. Drawing a shell model diagram and an energy diagram for hydrogen, and then using the. Explain bohr’s planetary model of the atom. Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher. Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher energy level, then immediately. Describe the triumphs and limits of bohr’s theory. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Although the bohr model is regarded as inaccurate and outdated, the model predicts the hydrogen atom well and provides good predictions on quantized energy levels.
1.8 The Bohr Theory of the Hydrogen Atom Chemistry LibreTexts
Bohr Model Energy Levels Although the bohr model is regarded as inaccurate and outdated, the model predicts the hydrogen atom well and provides good predictions on quantized energy levels. Each orbit was associated with an energy level. Describe the triumphs and limits of bohr’s theory. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. Calculating electron energy for levels n=1 to 3. Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher energy level, then immediately. Explain bohr’s planetary model of the atom. Although the bohr model is regarded as inaccurate and outdated, the model predicts the hydrogen atom well and provides good predictions on quantized energy levels. Drawing a shell model diagram and an energy diagram for hydrogen, and then using the. Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher. Niels bohr’s quantum model assumed that the electron could only orbit with certain allowed radii. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons.
From ar.inspiredpencil.com
Bohrs Model Energy Levels Bohr Model Energy Levels Drawing a shell model diagram and an energy diagram for hydrogen, and then using the. Although the bohr model is regarded as inaccurate and outdated, the model predicts the hydrogen atom well and provides good predictions on quantized energy levels. Explain bohr’s planetary model of the atom. Calculating electron energy for levels n=1 to 3. Niels bohr’s quantum model assumed. Bohr Model Energy Levels.
From scientifictutor.org
Chem Bohr Model and Electron Shells Bohr Model Energy Levels Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher energy level, then immediately. Although the bohr model is regarded as inaccurate and outdated, the model predicts the hydrogen atom well and provides good predictions on quantized energy levels. Describe the triumphs and limits of bohr’s theory.. Bohr Model Energy Levels.
From www.britannica.com
Chemical bonding Atomic structure and bonding Britannica Bohr Model Energy Levels Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher energy level, then immediately. Calculating electron energy for levels n=1 to 3. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Explain bohr’s planetary model of the atom. Although the bohr. Bohr Model Energy Levels.
From brainly.in
draw a diagram of bohrs model of an atom showing four energy shells Bohr Model Energy Levels Each orbit was associated with an energy level. Calculating electron energy for levels n=1 to 3. Describe the triumphs and limits of bohr’s theory. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Drawing a shell model diagram and an energy diagram for hydrogen, and then using the. Explain bohr’s planetary model of the. Bohr Model Energy Levels.
From ismlopez.weebly.com
Hydrogen bohr model ismlopez Bohr Model Energy Levels Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. Niels bohr’s quantum model assumed that the electron could only orbit with certain allowed radii. Calculating electron energy for levels n=1 to 3. Describe the triumphs and limits of bohr’s theory. Drawing a shell model diagram. Bohr Model Energy Levels.
From blogs.sch.gr
Άτομο Υποατομικά Σωματίδια Ατομική Θεωρία Οι αλλαγές στο ατομικό Bohr Model Energy Levels Niels bohr’s quantum model assumed that the electron could only orbit with certain allowed radii. Explain bohr’s planetary model of the atom. Each orbit was associated with an energy level. Although the bohr model is regarded as inaccurate and outdated, the model predicts the hydrogen atom well and provides good predictions on quantized energy levels. Calculating electron energy for levels. Bohr Model Energy Levels.
From www.pinterest.co.uk
Quantum Mechanics; Bohr Model, Energy Level Derivation and Calculations Bohr Model Energy Levels Although the bohr model is regarded as inaccurate and outdated, the model predicts the hydrogen atom well and provides good predictions on quantized energy levels. Each orbit was associated with an energy level. Explain bohr’s planetary model of the atom. Calculating electron energy for levels n=1 to 3. Bohr's model suggests that the atomic spectra of atoms is produced by. Bohr Model Energy Levels.
From 11de784a.github.io
Origin of Angular Momentum Quantization in Bohr's Model of Hydrogen Bohr Model Energy Levels Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Niels bohr’s quantum model assumed that the electron could only orbit with certain allowed radii. Describe the triumphs and limits of bohr’s theory. Each orbit was associated with an energy level. Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining. Bohr Model Energy Levels.
From www.slideserve.com
PPT THE BOHR MODEL OF THE ATOM PowerPoint Presentation, free download Bohr Model Energy Levels Explain bohr’s planetary model of the atom. Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher energy level, then immediately. Calculating electron energy for levels n=1 to 3. Drawing a shell model diagram and an energy diagram for hydrogen, and then using the. Bohr’s model of. Bohr Model Energy Levels.
From www.expii.com
Bohr's Atomic Model — Overview & Importance Expii Bohr Model Energy Levels Describe the triumphs and limits of bohr’s theory. Drawing a shell model diagram and an energy diagram for hydrogen, and then using the. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Calculating electron energy for levels n=1 to 3. Explain bohr’s planetary model of the atom. Bohr’s model of the hydrogen atom, proposed. Bohr Model Energy Levels.
From slideplayer.com
Bohr Model Energy levels ppt download Bohr Model Energy Levels Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher energy level, then immediately. Niels bohr’s quantum model assumed that the electron could only orbit with certain allowed radii. Explain bohr’s planetary model of the atom. Bohr’s model of the hydrogen atom, proposed by niels bohr in. Bohr Model Energy Levels.
From www.slideserve.com
PPT Chapter 5 The Periodic Law PowerPoint Presentation, free Bohr Model Energy Levels Calculating electron energy for levels n=1 to 3. Drawing a shell model diagram and an energy diagram for hydrogen, and then using the. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. Explain bohr’s planetary model of the atom. Each orbit was associated with an. Bohr Model Energy Levels.
From www.slideserve.com
PPT Outline the history of the atomic model. PowerPoint Presentation Bohr Model Energy Levels Describe the triumphs and limits of bohr’s theory. Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher. Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher energy level, then immediately. Bohr’s model. Bohr Model Energy Levels.
From favpng.com
Energy Level Hydrogen Atom Bohr Model Hydrogen Spectral Series, PNG Bohr Model Energy Levels Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher. Niels bohr’s quantum model assumed that the electron could only orbit with certain allowed radii. Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a. Bohr Model Energy Levels.
From favpng.com
Bohr Model Atom Diagram Energy Level Hydrogen, PNG, 800x768px, Bohr Bohr Model Energy Levels Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher energy level, then immediately. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that. Bohr Model Energy Levels.
From www.slideserve.com
PPT Bohr’s model of H atom PowerPoint Presentation, free download Bohr Model Energy Levels Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Although the bohr model is regarded as inaccurate and outdated, the model predicts the hydrogen atom well and provides good predictions on quantized energy levels. Niels bohr’s quantum model assumed that the electron could only orbit with certain allowed radii. Bohr's model suggests that the. Bohr Model Energy Levels.
From techschematic.com
The Ultimate Guide to Understanding Bohr Energy Level Diagrams Bohr Model Energy Levels Explain bohr’s planetary model of the atom. Niels bohr’s quantum model assumed that the electron could only orbit with certain allowed radii. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher.. Bohr Model Energy Levels.
From www.thoughtco.com
Bohr Model of the Atom Overview and Examples Bohr Model Energy Levels Explain bohr’s planetary model of the atom. Niels bohr’s quantum model assumed that the electron could only orbit with certain allowed radii. Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher energy level, then immediately. Each orbit was associated with an energy level. Calculating electron energy. Bohr Model Energy Levels.
From slidesharenow.blogspot.com
How To Draw A Bohr Model Of An Atom slideshare Bohr Model Energy Levels Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. Calculating electron energy for levels n=1 to 3. Drawing a shell model diagram and an energy diagram for hydrogen, and then using the. Each orbit was associated with an energy level. Bohr's model suggests that the. Bohr Model Energy Levels.
From www.globetrotterscience.com
Unit 8 Atomic Structure Bohr Model Energy Levels Drawing a shell model diagram and an energy diagram for hydrogen, and then using the. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. Describe the triumphs and limits of bohr’s theory. Bohr's model suggests that the atomic spectra of atoms is produced by electrons. Bohr Model Energy Levels.
From www.worksheetsplanet.com
Bohr's Atomic Model Bohr Model Energy Levels Drawing a shell model diagram and an energy diagram for hydrogen, and then using the. Describe the triumphs and limits of bohr’s theory. Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher. Explain bohr’s planetary model of the atom. Each orbit was associated with an energy. Bohr Model Energy Levels.
From quizlet.com
Diagram and label electron energy levels in Bohr's model. Quizlet Bohr Model Energy Levels Each orbit was associated with an energy level. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. Niels bohr’s quantum model assumed that the electron could only orbit with certain allowed radii. Bohr's model suggests that the atomic spectra of atoms is produced by electrons. Bohr Model Energy Levels.
From www.scienceabc.com
Niel Bohr's Atomic Theory Explained Bohr Model Energy Levels Calculating electron energy for levels n=1 to 3. Niels bohr’s quantum model assumed that the electron could only orbit with certain allowed radii. Explain bohr’s planetary model of the atom. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Describe the triumphs and limits of bohr’s theory. Bohr’s model of the hydrogen atom, proposed. Bohr Model Energy Levels.
From www.britannica.com
Atom Electrons, Orbitals, Energy Britannica Bohr Model Energy Levels Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher energy level, then immediately. Although the bohr model is regarded as inaccurate and outdated, the model predicts the hydrogen atom well and provides good predictions on quantized energy levels. Calculating electron energy for levels n=1 to 3.. Bohr Model Energy Levels.
From chem.libretexts.org
6.3 Line Spectra and the Bohr Model Chemistry LibreTexts Bohr Model Energy Levels Each orbit was associated with an energy level. Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher. Drawing a shell model diagram and an energy diagram for hydrogen, and then using the. Although the bohr model is regarded as inaccurate and outdated, the model predicts the. Bohr Model Energy Levels.
From www.expii.com
Bohr's Atomic Model — Overview & Importance Expii Bohr Model Energy Levels Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Each orbit was associated with an energy level. Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher. Drawing a shell model diagram and an energy diagram for hydrogen, and then using. Bohr Model Energy Levels.
From www.shutterstock.com
Energy Levels Atom Diagram Bohr Model Stock Vector (Royalty Free Bohr Model Energy Levels Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher. Drawing a shell model diagram and an energy diagram for hydrogen, and then using the. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Each orbit was associated with an energy. Bohr Model Energy Levels.
From newtondesk.com
Electron Configuration of Elements Chemistry Periodic Table Bohr Model Energy Levels Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher. Describe the triumphs and limits of bohr’s theory. Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher energy level, then immediately. Bohr’s model. Bohr Model Energy Levels.
From chem.libretexts.org
1.8 The Bohr Theory of the Hydrogen Atom Chemistry LibreTexts Bohr Model Energy Levels Describe the triumphs and limits of bohr’s theory. Drawing a shell model diagram and an energy diagram for hydrogen, and then using the. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a. Bohr Model Energy Levels.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps Bohr Model Energy Levels Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Explain bohr’s planetary model of the atom. Drawing a shell model diagram and an energy diagram for hydrogen, and then using the. Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher.. Bohr Model Energy Levels.
From www.thephysicsmill.com
Unreal Truths Matter Waves and the Bohr Model of the Atom Bohr Model Energy Levels Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher. Although the bohr model is regarded as inaccurate and outdated, the model predicts the hydrogen atom well and provides good predictions on quantized energy levels. Describe the triumphs and limits of bohr’s theory. Explain bohr’s planetary model. Bohr Model Energy Levels.
From cronodon.com
Atomic Models Bohr Model Energy Levels Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. Bohr's model suggests that the atomic spectra of atoms is produced by electrons. Bohr Model Energy Levels.
From www.carolina.com
Hydrogen Spectrum Activity Bohr Model Energy Levels Explain bohr’s planetary model of the atom. Drawing a shell model diagram and an energy diagram for hydrogen, and then using the. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of. Bohr Model Energy Levels.
From www.slideserve.com
PPT A t o m i c P h y s i c s What is the ATOM??? PowerPoint Bohr Model Energy Levels Describe the triumphs and limits of bohr’s theory. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. Drawing a shell model diagram and an energy diagram for hydrogen, and then. Bohr Model Energy Levels.
From classnotes.org.in
Bohr's Model of an Atom Chemistry, Class 11, Structure of Atom Bohr Model Energy Levels Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher. Explain bohr’s planetary model of the atom. Although the bohr model is regarded as inaccurate and outdated, the model predicts the hydrogen atom well and provides good predictions on quantized energy levels. Describe the triumphs and limits. Bohr Model Energy Levels.