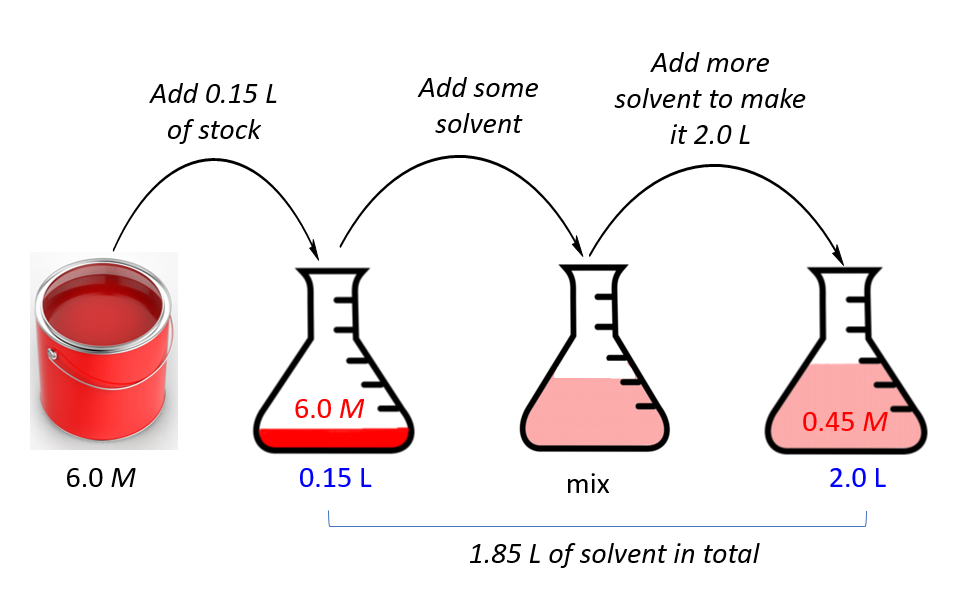
Dilution Uses . State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of. To dilute a solution means to add more solvent without the addition of more solute. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. Of course, the resulting solution is thoroughly mixed so as to. There are many ways of expressing concentration and dilution. Serial dilutions are used in microbiology to reduce bacterial. A concentrated solution contains a. Explanations and examples of common methods. Dilution is the process of weakening or deconcentrating a solution.
from general.chemistrysteps.com
Of course, the resulting solution is thoroughly mixed so as to. Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. To dilute a solution means to add more solvent without the addition of more solute. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the process of weakening or deconcentrating a solution. Explanations and examples of common methods. Concentration is the removal of solvent, which increases the concentration of. Serial dilutions are used in microbiology to reduce bacterial. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. There are many ways of expressing concentration and dilution.
Dilution of a Stock Solution and Calculations Based Morality
Dilution Uses State whether the concentration of a solution is directly or indirectly proportional to its volume. There are many ways of expressing concentration and dilution. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the process of weakening or deconcentrating a solution. A concentrated solution contains a. Explanations and examples of common methods. Concentration is the removal of solvent, which increases the concentration of. Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. Of course, the resulting solution is thoroughly mixed so as to. To dilute a solution means to add more solvent without the addition of more solute. Serial dilutions are used in microbiology to reduce bacterial.
From www.actioncleanup.com
Dilution Action’s Guide to Mixing the Right Solutions Dilution Uses Serial dilutions are used in microbiology to reduce bacterial. A concentrated solution contains a. Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. Concentration is the removal of solvent, which increases the concentration of. Dilution is the process of weakening or deconcentrating a solution. Dilution is. Dilution Uses.
From www.nowfoods.com
Essential Oil Dilution Guide Chart & Calculator NOW Foods Dilution Uses Serial dilutions are used in microbiology to reduce bacterial. A concentrated solution contains a. State whether the concentration of a solution is directly or indirectly proportional to its volume. Explanations and examples of common methods. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution is one in which there is. Dilution Uses.
From www.medicine.mcgill.ca
Serial Dilutions Dilution Uses Of course, the resulting solution is thoroughly mixed so as to. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. A concentrated solution contains a. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the process of weakening or deconcentrating a. Dilution Uses.
From www.pinterest.se
Dilution when solvent is added to dilute a solution, the number of Dilution Uses A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Of course, the resulting solution is thoroughly mixed so as to. State whether the concentration of a solution is directly or indirectly proportional to its volume. Explanations and examples of common methods. Dilution is the process of decreasing the concentration of. Dilution Uses.
From www.aromatics.com
Dilution Guidelines for Essential Oil Safety Dilution Uses Of course, the resulting solution is thoroughly mixed so as to. A concentrated solution contains a. Explanations and examples of common methods. Concentration is the removal of solvent, which increases the concentration of. Serial dilutions are used in microbiology to reduce bacterial. There are many ways of expressing concentration and dilution. Dilution is the addition of solvent, which decreases the. Dilution Uses.
From oilsporvida.blogspot.com
Oils Por Vida Guide to diluting essential oils for kids and babies Dilution Uses A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Serial dilutions are used in microbiology to reduce bacterial. State whether the concentration of a solution is directly or indirectly proportional to its volume. Of course, the resulting solution is thoroughly mixed so as to. To dilute a solution means to. Dilution Uses.
From ohlardy.com
Essential Oil Dilution Chart Printable Oh Lardy Dilution Uses Explanations and examples of common methods. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Serial dilutions are used in microbiology to reduce bacterial. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. There are many ways of expressing concentration and dilution. Dilution. Dilution Uses.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Uses Dilution is the process of weakening or deconcentrating a solution. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Concentration is the removal of solvent, which increases the concentration of. A concentrated solution contains a. To dilute a solution means to add more solvent without the addition of more solute.. Dilution Uses.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution Uses Serial dilutions are used in microbiology to reduce bacterial. Dilution is the process of weakening or deconcentrating a solution. A concentrated solution contains a. Explanations and examples of common methods. State whether the concentration of a solution is directly or indirectly proportional to its volume. To dilute a solution means to add more solvent without the addition of more solute.. Dilution Uses.
From www.pinterest.com
Coconut Oil Uses Proper dilution of essential oils is key to being Dilution Uses Concentration is the removal of solvent, which increases the concentration of. Of course, the resulting solution is thoroughly mixed so as to. A concentrated solution contains a. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution is one in which there is a relatively small amount of solute dissolved in. Dilution Uses.
From mommypotamus.com
Essential Oil Dilution Chart and Guidelines Dilution Uses Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Serial dilutions are used in microbiology to reduce bacterial. Dilution is the process of weakening or deconcentrating a solution. Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. Explanations and. Dilution Uses.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Uses Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. A concentrated solution contains a. Explanations and examples of common methods. State whether the concentration of a solution is directly or indirectly proportional to its. Dilution Uses.
From www.youtube.com
What Is Dilution? Chemistry Matters YouTube Dilution Uses A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Concentration is the removal of solvent, which increases the concentration of. Explanations and examples of common methods. Dilution is the process of weakening or deconcentrating a solution. A concentrated solution contains a. Of course, the resulting solution is thoroughly mixed so. Dilution Uses.
From magnoliasnaturals.org
Dilution guidelines for essential oils Magnolia's Naturals Dilution Uses To dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is thoroughly mixed so as to. A concentrated solution contains a. Explanations and examples of common methods. There are many ways of expressing concentration and dilution. Dilution is the addition of solvent, which decreases the concentration of the solute in. Dilution Uses.
From www.pinterest.com
How to Dilute Essential Oils A Complete Dilution Guide + Chart Dilution Uses Concentration is the removal of solvent, which increases the concentration of. A concentrated solution contains a. State whether the concentration of a solution is directly or indirectly proportional to its volume. To dilute a solution means to add more solvent without the addition of more solute. Dilution is the addition of solvent, which decreases the concentration of the solute in. Dilution Uses.
From www.youtube.com
Test Effectiveness of Disinfectants by Modified Use Dilution Method Dilution Uses Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. To dilute a solution means to add more solvent without the addition of more solute. Explanations and examples of common methods.. Dilution Uses.
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved Dilution Uses Concentration is the removal of solvent, which increases the concentration of. Serial dilutions are used in microbiology to reduce bacterial. Of course, the resulting solution is thoroughly mixed so as to. There are many ways of expressing concentration and dilution. Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. Dilution Uses.
From labrobot.com
Decimal dilutions Food microbiology Dilucup System Dilution Uses A concentrated solution contains a. There are many ways of expressing concentration and dilution. Of course, the resulting solution is thoroughly mixed so as to. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution. Dilution Uses.
From www.davinawellness.com
Dilution Guidelines Dilution Uses Explanations and examples of common methods. Of course, the resulting solution is thoroughly mixed so as to. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. To dilute a solution means to add more solvent without the addition of more solute. Dilution is the process of weakening or deconcentrating a solution. There are. Dilution Uses.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina Dilution Uses Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A concentrated solution contains a. To dilute a solution means to add more solvent without the addition of more solute. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the process of decreasing the concentration of. Dilution Uses.
From www.youtube.com
How to Perform a Bacterial Dilution Calculation YouTube Dilution Uses Serial dilutions are used in microbiology to reduce bacterial. To dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is thoroughly mixed so as to. There are many ways of expressing concentration and dilution. Explanations and examples of common methods. Concentration is the removal of solvent, which increases the concentration. Dilution Uses.
From www.youtube.com
Dilution Lab Results Chemistry Matters YouTube Dilution Uses Of course, the resulting solution is thoroughly mixed so as to. Serial dilutions are used in microbiology to reduce bacterial. A concentrated solution contains a. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which increases the concentration of. Dilution is the process of weakening or deconcentrating a. Dilution Uses.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality Dilution Uses State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. Concentration is the removal of solvent, which increases the concentration of. Dilution is the process of weakening or deconcentrating a solution. There. Dilution Uses.
From www.slideserve.com
PPT Preparing Solutions with Dilutions PowerPoint Presentation, free Dilution Uses There are many ways of expressing concentration and dilution. Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. Explanations and examples of common methods. A concentrated solution contains a. To dilute a solution means to add more solvent without the addition of more solute. Dilution is. Dilution Uses.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in Dilution Uses Dilution is the process of weakening or deconcentrating a solution. A concentrated solution contains a. To dilute a solution means to add more solvent without the addition of more solute. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Of course, the. Dilution Uses.
From www.pinterest.com.mx
The Essential Oils Dilution Chart Woman With Mind Essential oil Dilution Uses Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. A concentrated solution contains a. Explanations and examples of common methods. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of solvent, which decreases the concentration of. Dilution Uses.
From www.pinterest.com
Diluting Essential Oils Safely safe dilution guidelines for all ages Dilution Uses Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of. State whether the concentration of a solution is directly or indirectly proportional to its volume. A concentrated solution contains a. Serial dilutions are used in microbiology to reduce bacterial. Of course, the resulting. Dilution Uses.
From www.pinterest.co.uk
Robert Tisserand's safe essential oil dilution chart Are essential Dilution Uses Serial dilutions are used in microbiology to reduce bacterial. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Explanations and examples of common methods. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which increases the concentration of. There are many. Dilution Uses.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Uses Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the process of weakening or deconcentrating a solution. To dilute a solution means to add more solvent without the. Dilution Uses.
From www.pinterest.com
How To Dilute Essential Oils, Chart and PDF Got Oil Supplies Dilution Uses A concentrated solution contains a. Serial dilutions are used in microbiology to reduce bacterial. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Explanations and examples of common methods. There are many ways of expressing concentration and dilution. State whether the concentration of a solution is directly or indirectly proportional to its volume.. Dilution Uses.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Dilution Uses Dilution is the process of weakening or deconcentrating a solution. Serial dilutions are used in microbiology to reduce bacterial. Concentration is the removal of solvent, which increases the concentration of. Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. There are many ways of expressing concentration. Dilution Uses.
From www.pinterest.com
Using undiluted essential oils can lead to serious side effects. Here's Dilution Uses Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. Of course, the resulting solution is thoroughly mixed so as to. A concentrated solution contains a. There are many ways of expressing concentration and dilution. Concentration is the removal of solvent, which increases the concentration of. State. Dilution Uses.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Uses A concentrated solution contains a. Serial dilutions are used in microbiology to reduce bacterial. State whether the concentration of a solution is directly or indirectly proportional to its volume. To dilute a solution means to add more solvent without the addition of more solute. Explanations and examples of common methods. Dilution is the addition of solvent, which decreases the concentration. Dilution Uses.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Uses Serial dilutions are used in microbiology to reduce bacterial. Explanations and examples of common methods. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the process of weakening or deconcentrating a solution.. Dilution Uses.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilution Uses A concentrated solution contains a. Dilution is the process of weakening or deconcentrating a solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. Of course, the resulting solution is thoroughly mixed so as to. Serial dilutions are used in microbiology to reduce bacterial. A dilute solution is one in which there is a. Dilution Uses.