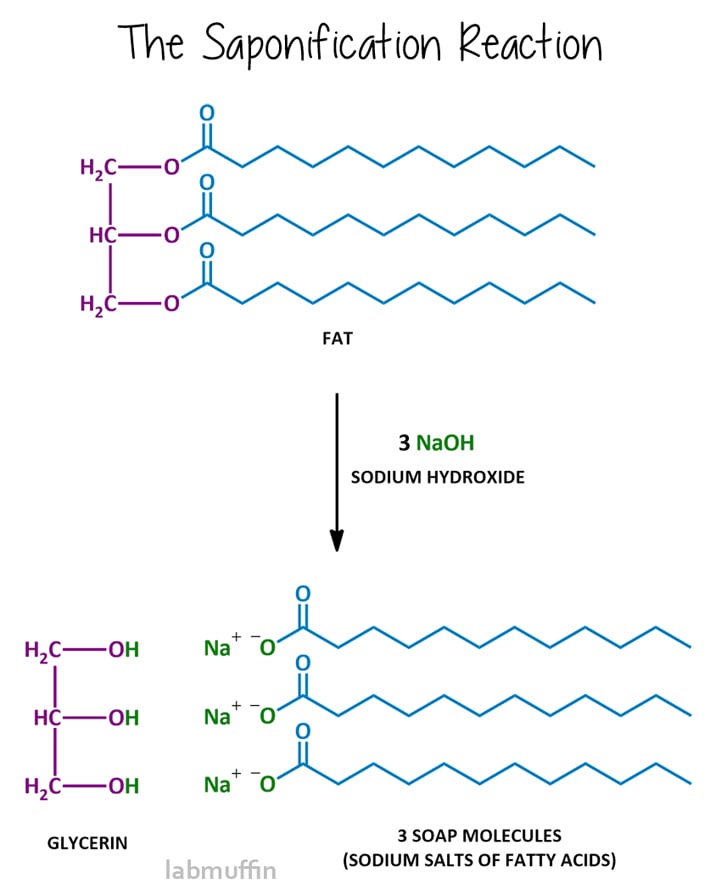
Soap And Chemistry Reaction . Learn about the chemistry of cleaning and how surfactants react with soil and water to clean everything. Note that each of these molecules has a nonpolar hydrocarbon chain, the tail, and a polar (often ionic) head group. The most common examples of such compounds are soaps and detergents, four of which are shown below. Saponification is the name of the chemical reaction that produces soap. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. Surfactants are a common ingredient in detergents and other cleaning products. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. The use of such compounds as cleaning agents is facilitated by their surfactant character, which lowers the surface tension of. The ancient roman tradition called for mixing rain water, potash and animal tallow (rendered form of. The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. The objective of this laboratory is to make lye soap via the saponification reaction. In the process, animal or vegetable fat is converted into soap (a fatty acid) and alcohol. In this collection of activities, students develop their understanding of key chemical ideas relating to soaps and detergents.
from labmuffin.com
The ancient roman tradition called for mixing rain water, potash and animal tallow (rendered form of. The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. The use of such compounds as cleaning agents is facilitated by their surfactant character, which lowers the surface tension of. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Saponification is the name of the chemical reaction that produces soap. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. The objective of this laboratory is to make lye soap via the saponification reaction. In this collection of activities, students develop their understanding of key chemical ideas relating to soaps and detergents. Surfactants are a common ingredient in detergents and other cleaning products.
Make Your Own Soap! Part 1 The Chemistry Behind Soap Making Lab
Soap And Chemistry Reaction Learn about the chemistry of cleaning and how surfactants react with soil and water to clean everything. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. The objective of this laboratory is to make lye soap via the saponification reaction. Saponification is the name of the chemical reaction that produces soap. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. Surfactants are a common ingredient in detergents and other cleaning products. The ancient roman tradition called for mixing rain water, potash and animal tallow (rendered form of. The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. In this collection of activities, students develop their understanding of key chemical ideas relating to soaps and detergents. Learn about the chemistry of cleaning and how surfactants react with soil and water to clean everything. Note that each of these molecules has a nonpolar hydrocarbon chain, the tail, and a polar (often ionic) head group. In the process, animal or vegetable fat is converted into soap (a fatty acid) and alcohol. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. The use of such compounds as cleaning agents is facilitated by their surfactant character, which lowers the surface tension of. The most common examples of such compounds are soaps and detergents, four of which are shown below.
From www.japudo.com.br
Chemistry Roberto Akira Soap And Chemistry Reaction In the process, animal or vegetable fat is converted into soap (a fatty acid) and alcohol. The use of such compounds as cleaning agents is facilitated by their surfactant character, which lowers the surface tension of. Learn about the chemistry of cleaning and how surfactants react with soil and water to clean everything. Saponification is the name of the chemical. Soap And Chemistry Reaction.
From www.shutterstock.com
Soap Reaction Water Vector Chemistry Diagram Stock Vector (Royalty Free Soap And Chemistry Reaction Saponification is the name of the chemical reaction that produces soap. Surfactants are a common ingredient in detergents and other cleaning products. The most common examples of such compounds are soaps and detergents, four of which are shown below. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process. Soap And Chemistry Reaction.
From studylib.net
Chemical Reactions Soap Making Soap And Chemistry Reaction Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. The ancient roman tradition called for mixing rain water, potash and animal tallow (rendered form of. In this collection of activities, students develop their understanding of key chemical ideas relating to soaps and detergents. Learn about. Soap And Chemistry Reaction.
From www.youtube.com
Chemistry 101 How does soap work? YouTube Soap And Chemistry Reaction In this collection of activities, students develop their understanding of key chemical ideas relating to soaps and detergents. Surfactants are a common ingredient in detergents and other cleaning products. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Saponification is the name of the chemical reaction that produces soap.. Soap And Chemistry Reaction.
From www.youtube.com
CLEANSING action of SOAP SURFACE CHEMISTRY CLASS12th NCERT YouTube Soap And Chemistry Reaction The most common examples of such compounds are soaps and detergents, four of which are shown below. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. In the process, animal or vegetable fat is converted into soap (a fatty acid) and alcohol. The ancient roman. Soap And Chemistry Reaction.
From www.thoughtco.com
Saponification Definition and Reaction Soap And Chemistry Reaction In this collection of activities, students develop their understanding of key chemical ideas relating to soaps and detergents. The ancient roman tradition called for mixing rain water, potash and animal tallow (rendered form of. Surfactants are a common ingredient in detergents and other cleaning products. Note that each of these molecules has a nonpolar hydrocarbon chain, the tail, and a. Soap And Chemistry Reaction.
From www.sharpestarena.com
Production of Soap Complete Project on Soap Making Soap And Chemistry Reaction In the process, animal or vegetable fat is converted into soap (a fatty acid) and alcohol. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. Note that each of these molecules has a nonpolar hydrocarbon chain, the tail, and a polar (often ionic) head group.. Soap And Chemistry Reaction.
From cosmosmagazine.com
The chemistry of soap Soap And Chemistry Reaction The ancient roman tradition called for mixing rain water, potash and animal tallow (rendered form of. Note that each of these molecules has a nonpolar hydrocarbon chain, the tail, and a polar (often ionic) head group. Surfactants are a common ingredient in detergents and other cleaning products. Saponification is the name of the chemical reaction that produces soap. Its fundamental. Soap And Chemistry Reaction.
From stock.adobe.com
Saponification equation, reaction of soap, chemistry equation of soap Soap And Chemistry Reaction Saponification is the name of the chemical reaction that produces soap. In the process, animal or vegetable fat is converted into soap (a fatty acid) and alcohol. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. Each soap molecule has a long hydrocarbon chain, sometimes. Soap And Chemistry Reaction.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation ID3090261 Soap And Chemistry Reaction Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. The use of such compounds as cleaning agents is facilitated by their surfactant character, which lowers the surface tension of. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'.. Soap And Chemistry Reaction.
From study.com
Saponification Definition, Reaction & Mechanism Lesson Soap And Chemistry Reaction The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. Saponification is the name of the chemical reaction that produces soap. In this collection of activities, students develop their understanding of key chemical ideas relating. Soap And Chemistry Reaction.
From historymeetsscience.blogspot.com
Tales of scientific journeys Soap making 101 Soap And Chemistry Reaction The use of such compounds as cleaning agents is facilitated by their surfactant character, which lowers the surface tension of. Learn about the chemistry of cleaning and how surfactants react with soil and water to clean everything. The most common examples of such compounds are soaps and detergents, four of which are shown below. Soaps are sodium or potassium fatty. Soap And Chemistry Reaction.
From www.pinterest.com
Chemical Reactions Chemical reactions, Soap, Soap making Soap And Chemistry Reaction In this collection of activities, students develop their understanding of key chemical ideas relating to soaps and detergents. In the process, animal or vegetable fat is converted into soap (a fatty acid) and alcohol. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. Soaps are. Soap And Chemistry Reaction.
From www.thoughtco.com
How Saponification Makes Soap Soap And Chemistry Reaction The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. Saponification is the name of the chemical reaction that produces soap. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. Surfactants are a common ingredient in. Soap And Chemistry Reaction.
From www.youtube.com
What is Saponification? Structure and Action of Soaps and Detergents Soap And Chemistry Reaction The use of such compounds as cleaning agents is facilitated by their surfactant character, which lowers the surface tension of. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Surfactants are a common ingredient in detergents and other cleaning products. Each soap molecule has a long hydrocarbon chain, sometimes. Soap And Chemistry Reaction.
From www.alamy.com
General formula of solid and liquid soap molecule. RCOONa, RCOOK Soap And Chemistry Reaction Learn about the chemistry of cleaning and how surfactants react with soil and water to clean everything. The objective of this laboratory is to make lye soap via the saponification reaction. The ancient roman tradition called for mixing rain water, potash and animal tallow (rendered form of. Its fundamental chemistry involves the combination of fats or oils with an alkaline. Soap And Chemistry Reaction.
From spmchemistry.blog.onlinetuition.com.my
The Making of Detergent SPM Chemistry Soap And Chemistry Reaction The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. Saponification is the name of the chemical reaction that produces soap. Note that each of these molecules has a nonpolar hydrocarbon chain, the tail, and a polar (often ionic) head group. Surfactants are a common ingredient in detergents and other cleaning. Soap And Chemistry Reaction.
From www.thoughtco.com
How Soap Works Soap And Chemistry Reaction Note that each of these molecules has a nonpolar hydrocarbon chain, the tail, and a polar (often ionic) head group. In the process, animal or vegetable fat is converted into soap (a fatty acid) and alcohol. The ancient roman tradition called for mixing rain water, potash and animal tallow (rendered form of. In this collection of activities, students develop their. Soap And Chemistry Reaction.
From spmchemistry.blog.onlinetuition.com.my
The Making of Detergent SPM Chemistry Soap And Chemistry Reaction Surfactants are a common ingredient in detergents and other cleaning products. Saponification is the name of the chemical reaction that produces soap. In the process, animal or vegetable fat is converted into soap (a fatty acid) and alcohol. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. Learn about the chemistry of cleaning. Soap And Chemistry Reaction.
From www.youtube.com
Cleansing action of soap Chemical reactions Chemistry YouTube Soap And Chemistry Reaction The use of such compounds as cleaning agents is facilitated by their surfactant character, which lowers the surface tension of. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. Note that each of these molecules has a nonpolar hydrocarbon chain, the tail, and a polar. Soap And Chemistry Reaction.
From www.slideserve.com
PPT Making Soap PowerPoint Presentation, free download ID1971971 Soap And Chemistry Reaction Note that each of these molecules has a nonpolar hydrocarbon chain, the tail, and a polar (often ionic) head group. The use of such compounds as cleaning agents is facilitated by their surfactant character, which lowers the surface tension of. The most common examples of such compounds are soaps and detergents, four of which are shown below. In this collection. Soap And Chemistry Reaction.
From www.slideshare.net
Soap and detergent ( chemistry folio form 5 ) Soap And Chemistry Reaction Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Learn about the chemistry of cleaning and how surfactants react with soil and water to clean everything. Saponification is the name of the chemical reaction that produces soap. Surfactants are a common ingredient in detergents and other cleaning products. The. Soap And Chemistry Reaction.
From ar.inspiredpencil.com
Preparation Of Soap In Chemistry Project Soap And Chemistry Reaction The objective of this laboratory is to make lye soap via the saponification reaction. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Saponification is the name of the chemical reaction that produces soap. The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in. Soap And Chemistry Reaction.
From studylib.net
Classification of Chemical Reactions The Soap Soap And Chemistry Reaction Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. Learn about the chemistry of cleaning and how surfactants react with soil and water to clean everything. Note that each. Soap And Chemistry Reaction.
From www.alamy.com
Soap Preparation Atomic representation of Saponification reaction Soap And Chemistry Reaction The most common examples of such compounds are soaps and detergents, four of which are shown below. Surfactants are a common ingredient in detergents and other cleaning products. Saponification is the name of the chemical reaction that produces soap. The ancient roman tradition called for mixing rain water, potash and animal tallow (rendered form of. Soaps are sodium or potassium. Soap And Chemistry Reaction.
From busy.org
Saponification 1/2 Soap And Chemistry Reaction In this collection of activities, students develop their understanding of key chemical ideas relating to soaps and detergents. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. The use of such compounds as cleaning agents is facilitated by their surfactant character, which lowers the surface tension of. The most common examples of such. Soap And Chemistry Reaction.
From www.shutterstock.com
Saponification Equation Reaction Soap Chemistry Equation Stock Soap And Chemistry Reaction The most common examples of such compounds are soaps and detergents, four of which are shown below. Saponification is the name of the chemical reaction that produces soap. The use of such compounds as cleaning agents is facilitated by their surfactant character, which lowers the surface tension of. The ancient roman tradition called for mixing rain water, potash and animal. Soap And Chemistry Reaction.
From www.coursehero.com
[Solved] Soap reactions. 1. Write the balanced equation for the Soap And Chemistry Reaction Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. The use of such compounds as cleaning agents is facilitated by their surfactant character, which lowers the surface tension of. In the process, animal or vegetable fat is converted into soap (a fatty acid) and alcohol. The ancient roman tradition called for mixing rain. Soap And Chemistry Reaction.
From www.youtube.com
[5.1] Cleansing action of soap YouTube Soap And Chemistry Reaction Saponification is the name of the chemical reaction that produces soap. The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. The use of such compounds as cleaning agents is facilitated by their surfactant character, which lowers the surface tension of. Its fundamental chemistry involves the combination of fats or oils. Soap And Chemistry Reaction.
From www.youtube.com
[5.1] Preparation of soap YouTube Soap And Chemistry Reaction Note that each of these molecules has a nonpolar hydrocarbon chain, the tail, and a polar (often ionic) head group. Learn about the chemistry of cleaning and how surfactants react with soil and water to clean everything. The most common examples of such compounds are soaps and detergents, four of which are shown below. In this collection of activities, students. Soap And Chemistry Reaction.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Soap Soap And Chemistry Reaction The objective of this laboratory is to make lye soap via the saponification reaction. In this collection of activities, students develop their understanding of key chemical ideas relating to soaps and detergents. The most common examples of such compounds are soaps and detergents, four of which are shown below. Its fundamental chemistry involves the combination of fats or oils with. Soap And Chemistry Reaction.
From www.scribd.com
The Chemical Reaction of Soap Making Chemistry Physical Sciences Soap And Chemistry Reaction Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. Saponification is the name of the chemical reaction that produces soap. The ancient roman tradition called for mixing rain water, potash and animal tallow (rendered form of. The objective of this laboratory is to make lye. Soap And Chemistry Reaction.
From labmuffin.com
Make Your Own Soap! Part 1 The Chemistry Behind Soap Making Lab Soap And Chemistry Reaction The use of such compounds as cleaning agents is facilitated by their surfactant character, which lowers the surface tension of. The ancient roman tradition called for mixing rain water, potash and animal tallow (rendered form of. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification.. Soap And Chemistry Reaction.
From busy.org
Saponification 1/2 Soap And Chemistry Reaction Saponification is the name of the chemical reaction that produces soap. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. Learn about the chemistry of cleaning and how surfactants react with soil and water to clean everything. Each soap molecule has a long hydrocarbon chain,. Soap And Chemistry Reaction.
From www.masterorganicchemistry.com
Basic Hydrolysis of Esters Saponification Soap And Chemistry Reaction The objective of this laboratory is to make lye soap via the saponification reaction. The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. Saponification is the name of the chemical reaction that produces soap. In this collection of activities, students develop their understanding of key chemical ideas relating to soaps. Soap And Chemistry Reaction.