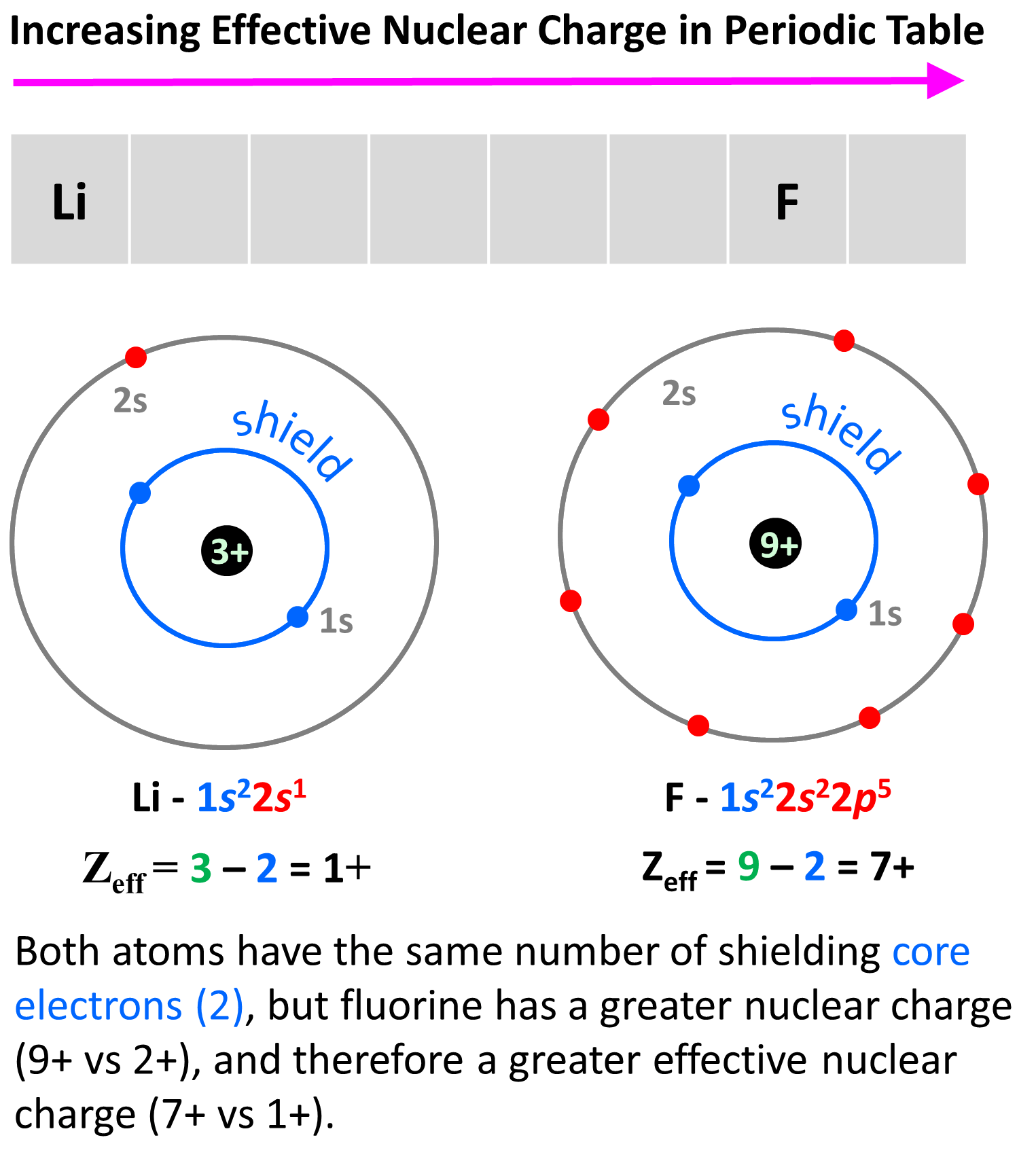
What Is The Connection Between Shielding And Effective Nuclear Charge . The effective nuclear charge affects the size of an atom, with a higher effective nuclear charge leading to a smaller atomic radius. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. This reduced charge is known as the effective nuclear charge. The effective nuclear charge may be approximated by the equation:. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge experienced by an outer electron due to the presence of inner electrons in the electron cloud. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons.
from general.chemistrysteps.com
The effective nuclear charge may be approximated by the equation:. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge experienced by an outer electron due to the presence of inner electrons in the electron cloud. Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. The effective nuclear charge affects the size of an atom, with a higher effective nuclear charge leading to a smaller atomic radius. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. This reduced charge is known as the effective nuclear charge.
Effective Nuclear Charge Chemistry Steps
What Is The Connection Between Shielding And Effective Nuclear Charge The effective nuclear charge affects the size of an atom, with a higher effective nuclear charge leading to a smaller atomic radius. Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. The effective nuclear charge may be approximated by the equation:. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The effective nuclear charge affects the size of an atom, with a higher effective nuclear charge leading to a smaller atomic radius. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge experienced by an outer electron due to the presence of inner electrons in the electron cloud. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. This reduced charge is known as the effective nuclear charge.
From www.slideserve.com
PPT Quantum Theory PowerPoint Presentation, free download ID1185278 What Is The Connection Between Shielding And Effective Nuclear Charge The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The effective nuclear charge may be approximated by the equation:. The effective nuclear charge affects the size of an atom, with a higher effective nuclear charge leading to a smaller atomic radius. Overall, the outer electrons experience a lower force and a reduced. What Is The Connection Between Shielding And Effective Nuclear Charge.
From pediaa.com
What is the Difference Between Effective Nuclear Charge and Shielding What Is The Connection Between Shielding And Effective Nuclear Charge The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The effective nuclear charge affects the size of an atom, with a higher effective nuclear charge leading to a smaller atomic radius. Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the. What Is The Connection Between Shielding And Effective Nuclear Charge.
From www.youtube.com
Nuclear Charge, Shielding Effect & Effective Nuclear Charge (Singapore What Is The Connection Between Shielding And Effective Nuclear Charge The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge experienced by an outer electron due to the presence of inner electrons in. What Is The Connection Between Shielding And Effective Nuclear Charge.
From www.youtube.com
Effective Nuclear Charge Chemistry Tutorial YouTube What Is The Connection Between Shielding And Effective Nuclear Charge This reduced charge is known as the effective nuclear charge. The effective nuclear charge affects the size of an atom, with a higher effective nuclear charge leading to a smaller atomic radius. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective. What Is The Connection Between Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT Chapter 6 Periodic Trends PowerPoint Presentation, free download What Is The Connection Between Shielding And Effective Nuclear Charge Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge experienced by an outer electron due to the presence of inner electrons in the electron cloud. This reduced charge is known as the effective nuclear charge. The effective nuclear charge. What Is The Connection Between Shielding And Effective Nuclear Charge.
From www.youtube.com
Nuclear Charge and Effective Nuclear Charge YouTube What Is The Connection Between Shielding And Effective Nuclear Charge This reduced charge is known as the effective nuclear charge. The effective nuclear charge may be approximated by the equation:. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The effective nuclear charge affects the size of an atom, with a higher effective nuclear charge leading to a smaller atomic radius. The. What Is The Connection Between Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT The Modern Periodic Table PowerPoint Presentation, free download What Is The Connection Between Shielding And Effective Nuclear Charge The effective nuclear charge may be approximated by the equation:. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge experienced by an outer electron due to the presence of inner electrons in the electron cloud. The concept of electron. What Is The Connection Between Shielding And Effective Nuclear Charge.
From chem.libretexts.org
1.15 Effective Nuclear Charge and Shielding Chemistry LibreTexts What Is The Connection Between Shielding And Effective Nuclear Charge The effective nuclear charge may be approximated by the equation:. This reduced charge is known as the effective nuclear charge. The effective nuclear charge affects the size of an atom, with a higher effective nuclear charge leading to a smaller atomic radius. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by. What Is The Connection Between Shielding And Effective Nuclear Charge.
From www.youtube.com
How To Calculate The Effective Nuclear Charge of an Electron YouTube What Is The Connection Between Shielding And Effective Nuclear Charge The effective nuclear charge may be approximated by the equation:. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge experienced by an outer electron due to the presence of inner electrons in the electron cloud. The effective nuclear charge. What Is The Connection Between Shielding And Effective Nuclear Charge.
From www.breakingatom.com
Nuclear Charge What Is The Connection Between Shielding And Effective Nuclear Charge Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge experienced by an outer electron due to the presence of inner electrons in the electron cloud. The effective nuclear charge affects the size of an atom, with a higher effective. What Is The Connection Between Shielding And Effective Nuclear Charge.
From slideplayer.com
Periodic Trends. ppt download What Is The Connection Between Shielding And Effective Nuclear Charge The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The effective nuclear charge affects the size of an atom, with a higher effective nuclear charge leading to a smaller atomic radius. This reduced charge is known as the effective nuclear charge. The concept of electron shielding, in which intervening electrons act to. What Is The Connection Between Shielding And Effective Nuclear Charge.
From www.youtube.com
Shielding and Effective Nuclear Charge YouTube What Is The Connection Between Shielding And Effective Nuclear Charge The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The effective nuclear charge may be approximated by the equation:. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge. What Is The Connection Between Shielding And Effective Nuclear Charge.
From www.youtube.com
effective nuclear charge YouTube What Is The Connection Between Shielding And Effective Nuclear Charge The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge experienced by an outer electron due to the presence of inner electrons in. What Is The Connection Between Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT Lecture 6 PowerPoint Presentation, free download ID2643994 What Is The Connection Between Shielding And Effective Nuclear Charge Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. The effective nuclear charge affects the size of an atom, with a higher effective nuclear charge leading to a smaller atomic radius. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The. What Is The Connection Between Shielding And Effective Nuclear Charge.
From general.chemistrysteps.com
Effective Nuclear Charge Chemistry Steps What Is The Connection Between Shielding And Effective Nuclear Charge The effective nuclear charge affects the size of an atom, with a higher effective nuclear charge leading to a smaller atomic radius. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom,. What Is The Connection Between Shielding And Effective Nuclear Charge.
From www.youtube.com
S3.1.3 Electron shielding and effective nuclear charge YouTube What Is The Connection Between Shielding And Effective Nuclear Charge This reduced charge is known as the effective nuclear charge. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge experienced by an outer electron due to the presence of inner electrons in the electron cloud. Overall, the outer electrons. What Is The Connection Between Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT Chapter 7 Periodic Properties of the Elements PowerPoint What Is The Connection Between Shielding And Effective Nuclear Charge The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge experienced by an outer electron due to the presence of. What Is The Connection Between Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT Transition Metals (Ligands) PowerPoint Presentation ID1273978 What Is The Connection Between Shielding And Effective Nuclear Charge This reduced charge is known as the effective nuclear charge. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge. What Is The Connection Between Shielding And Effective Nuclear Charge.
From general.chemistrysteps.com
Effective Nuclear Charge Chemistry Steps What Is The Connection Between Shielding And Effective Nuclear Charge The effective nuclear charge may be approximated by the equation:. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge experienced by an outer electron due to the presence of inner electrons in the electron cloud. This reduced charge is. What Is The Connection Between Shielding And Effective Nuclear Charge.
From www.numerade.com
SOLVEDDefine shielding and effective nuclear charge. What is the What Is The Connection Between Shielding And Effective Nuclear Charge The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The effective nuclear charge may be approximated by the equation:. Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. The effective nuclear charge affects the size of an atom,. What Is The Connection Between Shielding And Effective Nuclear Charge.
From favpng.com
Effective Nuclear Charge Shielding Effect Atomic Nucleus Atomic Number What Is The Connection Between Shielding And Effective Nuclear Charge The effective nuclear charge may be approximated by the equation:. This reduced charge is known as the effective nuclear charge. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge experienced by an outer electron due to the presence of. What Is The Connection Between Shielding And Effective Nuclear Charge.
From gamma.app
Understanding Shielding Effect and Effective Nuclear Charge What Is The Connection Between Shielding And Effective Nuclear Charge This reduced charge is known as the effective nuclear charge. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge experienced by an outer electron due to the presence of inner electrons in the electron cloud. The effective nuclear charge. What Is The Connection Between Shielding And Effective Nuclear Charge.
From www.youtube.com
Shielding Effect and Effective Nuclear Charge YouTube What Is The Connection Between Shielding And Effective Nuclear Charge Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. The effective nuclear charge may be approximated by the equation:. This reduced charge is known as the effective nuclear charge. The effective nuclear charge affects the size of an atom, with a higher effective nuclear charge leading to a smaller. What Is The Connection Between Shielding And Effective Nuclear Charge.
From slideplayer.com
Effective Nuclear Charge, Shielding, and Trends on the Periodic Table What Is The Connection Between Shielding And Effective Nuclear Charge The effective nuclear charge affects the size of an atom, with a higher effective nuclear charge leading to a smaller atomic radius. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. This. What Is The Connection Between Shielding And Effective Nuclear Charge.
From www.youtube.com
Effective Nuclear Charge for Intro Chem Students.wmv YouTube What Is The Connection Between Shielding And Effective Nuclear Charge The effective nuclear charge affects the size of an atom, with a higher effective nuclear charge leading to a smaller atomic radius. Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The. What Is The Connection Between Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT Atomic Radii PowerPoint Presentation, free download ID6448698 What Is The Connection Between Shielding And Effective Nuclear Charge The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. The effective nuclear charge affects the size of an atom, with a higher effective nuclear charge leading to a smaller. What Is The Connection Between Shielding And Effective Nuclear Charge.
From ar.inspiredpencil.com
Shielding Effect Electrons What Is The Connection Between Shielding And Effective Nuclear Charge The effective nuclear charge may be approximated by the equation:. This reduced charge is known as the effective nuclear charge. Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the. What Is The Connection Between Shielding And Effective Nuclear Charge.
From socratic.org
How are shielding effect and atomic radius related? Socratic What Is The Connection Between Shielding And Effective Nuclear Charge The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The effective nuclear charge may be approximated by the equation:. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. This reduced charge is known as the effective nuclear charge. Effective nuclear charge. What Is The Connection Between Shielding And Effective Nuclear Charge.
From www.vedantu.com
Shielding Effect and Effective Nuclear Charge Important Concepts for JEE What Is The Connection Between Shielding And Effective Nuclear Charge The effective nuclear charge may be approximated by the equation:. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge experienced by an. What Is The Connection Between Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT Periodic Patterns PowerPoint Presentation ID6476349 What Is The Connection Between Shielding And Effective Nuclear Charge The effective nuclear charge may be approximated by the equation:. Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. This reduced charge is known as the effective nuclear charge. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The effective nuclear. What Is The Connection Between Shielding And Effective Nuclear Charge.
From www.youtube.com
Difference between Nuclear Charge and Effective Nuclear Charge What Is The Connection Between Shielding And Effective Nuclear Charge The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is. What Is The Connection Between Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT Why “periodic?” PowerPoint Presentation, free download ID1779615 What Is The Connection Between Shielding And Effective Nuclear Charge The effective nuclear charge affects the size of an atom, with a higher effective nuclear charge leading to a smaller atomic radius. The effective nuclear charge may be approximated by the equation:. This reduced charge is known as the effective nuclear charge. Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the. What Is The Connection Between Shielding And Effective Nuclear Charge.
From www.youtube.com
Shielding Effect and Effective Nuclear Charge YouTube What Is The Connection Between Shielding And Effective Nuclear Charge This reduced charge is known as the effective nuclear charge. The effective nuclear charge affects the size of an atom, with a higher effective nuclear charge leading to a smaller atomic radius. The effective nuclear charge may be approximated by the equation:. Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the. What Is The Connection Between Shielding And Effective Nuclear Charge.
From pediaa.com
Difference Between Nuclear Charge and Effective Nuclear Charge What Is The Connection Between Shielding And Effective Nuclear Charge Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge experienced by an outer electron due to the presence of inner electrons in the electron cloud. The effective nuclear charge may be approximated by the equation:. The effective nuclear charge. What Is The Connection Between Shielding And Effective Nuclear Charge.
From byjus.com
Explain effective nuclear charge shielding effect Factor on which What Is The Connection Between Shielding And Effective Nuclear Charge The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is. What Is The Connection Between Shielding And Effective Nuclear Charge.