Histamine Release With Cisatracurium . Cisatracurium was developed primarily for anaesthetic purposes in order to attempt to resolve some of the problems associated with. Histamine release can be minimised by administering slowly or in. This activity reviews indications, mechanism of action, administration, contraindications, monitoring, and toxicity associated with cisatracurium and the role of. Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant histamine release, producing tachycardia, hypotension, and skin flushing. The purposes of our study were to compare equipotent doses of vecuronium and cisatracurium for chemical, systemic, or. Cardiovascular effects are associated with significant histamine release; Generally, insignificant clinically but may occur with all the nmbas in large bolus doses, administered rapidly.
from www.semanticscholar.org
Generally, insignificant clinically but may occur with all the nmbas in large bolus doses, administered rapidly. This activity reviews indications, mechanism of action, administration, contraindications, monitoring, and toxicity associated with cisatracurium and the role of. Histamine release can be minimised by administering slowly or in. Cisatracurium was developed primarily for anaesthetic purposes in order to attempt to resolve some of the problems associated with. The purposes of our study were to compare equipotent doses of vecuronium and cisatracurium for chemical, systemic, or. Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant histamine release, producing tachycardia, hypotension, and skin flushing. Cardiovascular effects are associated with significant histamine release;
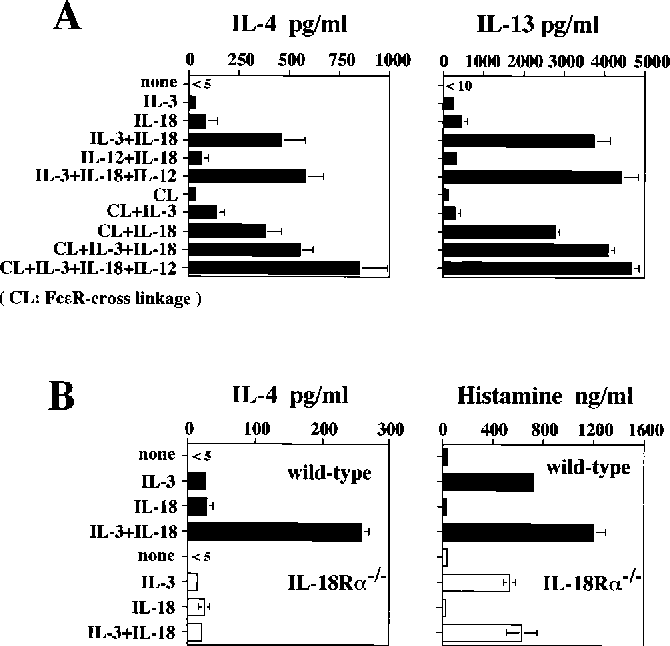
Figure 1 from IL18, although antiallergic when administered with IL12
Histamine Release With Cisatracurium Histamine release can be minimised by administering slowly or in. The purposes of our study were to compare equipotent doses of vecuronium and cisatracurium for chemical, systemic, or. Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant histamine release, producing tachycardia, hypotension, and skin flushing. Generally, insignificant clinically but may occur with all the nmbas in large bolus doses, administered rapidly. Histamine release can be minimised by administering slowly or in. Cardiovascular effects are associated with significant histamine release; This activity reviews indications, mechanism of action, administration, contraindications, monitoring, and toxicity associated with cisatracurium and the role of. Cisatracurium was developed primarily for anaesthetic purposes in order to attempt to resolve some of the problems associated with.
From www.semanticscholar.org
Figure 1 from IL18, although antiallergic when administered with IL12 Histamine Release With Cisatracurium Cardiovascular effects are associated with significant histamine release; The purposes of our study were to compare equipotent doses of vecuronium and cisatracurium for chemical, systemic, or. This activity reviews indications, mechanism of action, administration, contraindications, monitoring, and toxicity associated with cisatracurium and the role of. Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant. Histamine Release With Cisatracurium.
From www.researchgate.net
Constitutive histamine release in HLMCHASMC coculture and it's Histamine Release With Cisatracurium Cardiovascular effects are associated with significant histamine release; Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant histamine release, producing tachycardia, hypotension, and skin flushing. Cisatracurium was developed primarily for anaesthetic purposes in order to attempt to resolve some of the problems associated with. The purposes of our study were to compare equipotent doses. Histamine Release With Cisatracurium.
From drbeckycampbell.com
Understanding Histamine Intolerance Dr Becky Campbell Histamine Release With Cisatracurium Cardiovascular effects are associated with significant histamine release; The purposes of our study were to compare equipotent doses of vecuronium and cisatracurium for chemical, systemic, or. Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant histamine release, producing tachycardia, hypotension, and skin flushing. This activity reviews indications, mechanism of action, administration, contraindications, monitoring, and. Histamine Release With Cisatracurium.
From www.slideserve.com
PPT CLINICAL PHARMACOLOGY OF NEUROMUSCULAR BLOCKING AGENTS PowerPoint Histamine Release With Cisatracurium Histamine release can be minimised by administering slowly or in. Cardiovascular effects are associated with significant histamine release; This activity reviews indications, mechanism of action, administration, contraindications, monitoring, and toxicity associated with cisatracurium and the role of. Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant histamine release, producing tachycardia, hypotension, and skin flushing.. Histamine Release With Cisatracurium.
From www.semanticscholar.org
Figure 1 from Impact of IL32 on Histamine Release by Human Derived Histamine Release With Cisatracurium Histamine release can be minimised by administering slowly or in. Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant histamine release, producing tachycardia, hypotension, and skin flushing. Cardiovascular effects are associated with significant histamine release; This activity reviews indications, mechanism of action, administration, contraindications, monitoring, and toxicity associated with cisatracurium and the role of.. Histamine Release With Cisatracurium.
From slideplayer.com
Non depolarizing muscle relaxant ppt download Histamine Release With Cisatracurium Histamine release can be minimised by administering slowly or in. Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant histamine release, producing tachycardia, hypotension, and skin flushing. The purposes of our study were to compare equipotent doses of vecuronium and cisatracurium for chemical, systemic, or. Cisatracurium was developed primarily for anaesthetic purposes in order. Histamine Release With Cisatracurium.
From katedaugherty.com
Histamine Intolerance A Dietary Overview Kate Daugherty Histamine Release With Cisatracurium The purposes of our study were to compare equipotent doses of vecuronium and cisatracurium for chemical, systemic, or. Histamine release can be minimised by administering slowly or in. This activity reviews indications, mechanism of action, administration, contraindications, monitoring, and toxicity associated with cisatracurium and the role of. Cardiovascular effects are associated with significant histamine release; Generally, insignificant clinically but may. Histamine Release With Cisatracurium.
From www.researchgate.net
1 Triggers of mast cell histamine release Download Scientific Diagram Histamine Release With Cisatracurium Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant histamine release, producing tachycardia, hypotension, and skin flushing. Cardiovascular effects are associated with significant histamine release; Histamine release can be minimised by administering slowly or in. This activity reviews indications, mechanism of action, administration, contraindications, monitoring, and toxicity associated with cisatracurium and the role of.. Histamine Release With Cisatracurium.
From www.researchgate.net
Histamineproducing cells and stimuli that trigger histamine release Histamine Release With Cisatracurium Generally, insignificant clinically but may occur with all the nmbas in large bolus doses, administered rapidly. Histamine release can be minimised by administering slowly or in. The purposes of our study were to compare equipotent doses of vecuronium and cisatracurium for chemical, systemic, or. Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant histamine. Histamine Release With Cisatracurium.
From slideplayer.com
Pharmacology of general anesthetics ppt download Histamine Release With Cisatracurium Cardiovascular effects are associated with significant histamine release; This activity reviews indications, mechanism of action, administration, contraindications, monitoring, and toxicity associated with cisatracurium and the role of. Cisatracurium was developed primarily for anaesthetic purposes in order to attempt to resolve some of the problems associated with. Generally, insignificant clinically but may occur with all the nmbas in large bolus doses,. Histamine Release With Cisatracurium.
From www.researchgate.net
Classification of histaminerelease responses by severity Download Table Histamine Release With Cisatracurium Cisatracurium was developed primarily for anaesthetic purposes in order to attempt to resolve some of the problems associated with. This activity reviews indications, mechanism of action, administration, contraindications, monitoring, and toxicity associated with cisatracurium and the role of. Cardiovascular effects are associated with significant histamine release; Generally, insignificant clinically but may occur with all the nmbas in large bolus doses,. Histamine Release With Cisatracurium.
From www.nejm.org
Spontaneous Release of Histamine from Basophils and HistamineReleasing Histamine Release With Cisatracurium Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant histamine release, producing tachycardia, hypotension, and skin flushing. Cardiovascular effects are associated with significant histamine release; The purposes of our study were to compare equipotent doses of vecuronium and cisatracurium for chemical, systemic, or. Histamine release can be minimised by administering slowly or in. Generally,. Histamine Release With Cisatracurium.
From www.researchgate.net
(PDF) Recovery Profile of Atracurium versus Cisatracurium Histamine Release With Cisatracurium The purposes of our study were to compare equipotent doses of vecuronium and cisatracurium for chemical, systemic, or. Histamine release can be minimised by administering slowly or in. Generally, insignificant clinically but may occur with all the nmbas in large bolus doses, administered rapidly. This activity reviews indications, mechanism of action, administration, contraindications, monitoring, and toxicity associated with cisatracurium and. Histamine Release With Cisatracurium.
From www.academia.edu
(PDF) The Lack of Histamine Release with Cisatracurium A DoubleBlind Histamine Release With Cisatracurium The purposes of our study were to compare equipotent doses of vecuronium and cisatracurium for chemical, systemic, or. Histamine release can be minimised by administering slowly or in. Generally, insignificant clinically but may occur with all the nmbas in large bolus doses, administered rapidly. Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant histamine. Histamine Release With Cisatracurium.
From www.researchgate.net
A model of the histamine recycling pathway. Histamine is de novo Histamine Release With Cisatracurium Generally, insignificant clinically but may occur with all the nmbas in large bolus doses, administered rapidly. Histamine release can be minimised by administering slowly or in. This activity reviews indications, mechanism of action, administration, contraindications, monitoring, and toxicity associated with cisatracurium and the role of. Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant. Histamine Release With Cisatracurium.
From medicalverge.in
Histamine role and actions MedicalVerge Histamine Release With Cisatracurium Generally, insignificant clinically but may occur with all the nmbas in large bolus doses, administered rapidly. The purposes of our study were to compare equipotent doses of vecuronium and cisatracurium for chemical, systemic, or. This activity reviews indications, mechanism of action, administration, contraindications, monitoring, and toxicity associated with cisatracurium and the role of. Cardiovascular effects are associated with significant histamine. Histamine Release With Cisatracurium.
From www.pnas.org
IL18, although antiallergic when administered with IL12, stimulates Histamine Release With Cisatracurium Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant histamine release, producing tachycardia, hypotension, and skin flushing. Generally, insignificant clinically but may occur with all the nmbas in large bolus doses, administered rapidly. Cisatracurium was developed primarily for anaesthetic purposes in order to attempt to resolve some of the problems associated with. The purposes. Histamine Release With Cisatracurium.
From www.researchgate.net
Histamine release from activated and induced RBL2H3cells treated Histamine Release With Cisatracurium Cisatracurium was developed primarily for anaesthetic purposes in order to attempt to resolve some of the problems associated with. Histamine release can be minimised by administering slowly or in. Cardiovascular effects are associated with significant histamine release; Generally, insignificant clinically but may occur with all the nmbas in large bolus doses, administered rapidly. The purposes of our study were to. Histamine Release With Cisatracurium.
From www.researchgate.net
Total histamine release after MCP1 (10 and 20 ng/50 µl) and FMLP (106 Histamine Release With Cisatracurium Generally, insignificant clinically but may occur with all the nmbas in large bolus doses, administered rapidly. The purposes of our study were to compare equipotent doses of vecuronium and cisatracurium for chemical, systemic, or. Cisatracurium was developed primarily for anaesthetic purposes in order to attempt to resolve some of the problems associated with. This activity reviews indications, mechanism of action,. Histamine Release With Cisatracurium.
From www.researchgate.net
Histamine release in RBL2H3 cells, MRGPRX2RBL2H3 cells, and RPMCs Histamine Release With Cisatracurium Histamine release can be minimised by administering slowly or in. Generally, insignificant clinically but may occur with all the nmbas in large bolus doses, administered rapidly. The purposes of our study were to compare equipotent doses of vecuronium and cisatracurium for chemical, systemic, or. Cisatracurium was developed primarily for anaesthetic purposes in order to attempt to resolve some of the. Histamine Release With Cisatracurium.
From www.semanticscholar.org
The histaminergic network in the brain basic organization and role in Histamine Release With Cisatracurium Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant histamine release, producing tachycardia, hypotension, and skin flushing. This activity reviews indications, mechanism of action, administration, contraindications, monitoring, and toxicity associated with cisatracurium and the role of. Cardiovascular effects are associated with significant histamine release; The purposes of our study were to compare equipotent doses. Histamine Release With Cisatracurium.
From www.youtube.com
Histamine synthesis, storage, release and metabolism Histamine Release With Cisatracurium Generally, insignificant clinically but may occur with all the nmbas in large bolus doses, administered rapidly. This activity reviews indications, mechanism of action, administration, contraindications, monitoring, and toxicity associated with cisatracurium and the role of. Histamine release can be minimised by administering slowly or in. Cardiovascular effects are associated with significant histamine release; Intubating doses of atracurium (0.5 mg/kg or. Histamine Release With Cisatracurium.
From www.slideserve.com
PPT Neuromuscular Relaxants + Reversal Agents PowerPoint Presentation Histamine Release With Cisatracurium Histamine release can be minimised by administering slowly or in. Generally, insignificant clinically but may occur with all the nmbas in large bolus doses, administered rapidly. Cisatracurium was developed primarily for anaesthetic purposes in order to attempt to resolve some of the problems associated with. Cardiovascular effects are associated with significant histamine release; This activity reviews indications, mechanism of action,. Histamine Release With Cisatracurium.
From journals.lww.com
The Lack of Histamine Release with Cisatracurium A DoubleB Histamine Release With Cisatracurium The purposes of our study were to compare equipotent doses of vecuronium and cisatracurium for chemical, systemic, or. Histamine release can be minimised by administering slowly or in. Generally, insignificant clinically but may occur with all the nmbas in large bolus doses, administered rapidly. Cisatracurium was developed primarily for anaesthetic purposes in order to attempt to resolve some of the. Histamine Release With Cisatracurium.
From www.researchgate.net
SLC15A4 regulates histamine release in vivo. (A) Serum histamine levels Histamine Release With Cisatracurium Generally, insignificant clinically but may occur with all the nmbas in large bolus doses, administered rapidly. This activity reviews indications, mechanism of action, administration, contraindications, monitoring, and toxicity associated with cisatracurium and the role of. Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant histamine release, producing tachycardia, hypotension, and skin flushing. Cisatracurium was. Histamine Release With Cisatracurium.
From www.slideserve.com
PPT Neuromuscular Blocking Agents PowerPoint Presentation, free Histamine Release With Cisatracurium Generally, insignificant clinically but may occur with all the nmbas in large bolus doses, administered rapidly. Histamine release can be minimised by administering slowly or in. Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant histamine release, producing tachycardia, hypotension, and skin flushing. Cisatracurium was developed primarily for anaesthetic purposes in order to attempt. Histamine Release With Cisatracurium.
From www.frontiersin.org
Frontiers Histamine induced high mobility group box1 release from Histamine Release With Cisatracurium Cisatracurium was developed primarily for anaesthetic purposes in order to attempt to resolve some of the problems associated with. This activity reviews indications, mechanism of action, administration, contraindications, monitoring, and toxicity associated with cisatracurium and the role of. Histamine release can be minimised by administering slowly or in. Cardiovascular effects are associated with significant histamine release; Intubating doses of atracurium. Histamine Release With Cisatracurium.
From www.jneurosci.org
Release of Histamine Reveals Distal and Histamine Release With Cisatracurium The purposes of our study were to compare equipotent doses of vecuronium and cisatracurium for chemical, systemic, or. Generally, insignificant clinically but may occur with all the nmbas in large bolus doses, administered rapidly. Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant histamine release, producing tachycardia, hypotension, and skin flushing. Cardiovascular effects are. Histamine Release With Cisatracurium.
From myhappygenes.com
Histamine Information Page Activate Your Happy Genes Histamine Release With Cisatracurium The purposes of our study were to compare equipotent doses of vecuronium and cisatracurium for chemical, systemic, or. Cisatracurium was developed primarily for anaesthetic purposes in order to attempt to resolve some of the problems associated with. Histamine release can be minimised by administering slowly or in. Cardiovascular effects are associated with significant histamine release; This activity reviews indications, mechanism. Histamine Release With Cisatracurium.
From www.researchgate.net
IMP reduced degranulation, histamine release, and intracellular calcium Histamine Release With Cisatracurium Histamine release can be minimised by administering slowly or in. This activity reviews indications, mechanism of action, administration, contraindications, monitoring, and toxicity associated with cisatracurium and the role of. Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant histamine release, producing tachycardia, hypotension, and skin flushing. Generally, insignificant clinically but may occur with all. Histamine Release With Cisatracurium.
From steptohealth.com
Histamine Synthesis, Release and Functions Step To Health Histamine Release With Cisatracurium Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant histamine release, producing tachycardia, hypotension, and skin flushing. This activity reviews indications, mechanism of action, administration, contraindications, monitoring, and toxicity associated with cisatracurium and the role of. Histamine release can be minimised by administering slowly or in. Generally, insignificant clinically but may occur with all. Histamine Release With Cisatracurium.
From www.slideserve.com
PPT CLINICAL PHARMACOLOGY OF NEUROMUSCULAR BLOCKING AGENTS PowerPoint Histamine Release With Cisatracurium Histamine release can be minimised by administering slowly or in. Cardiovascular effects are associated with significant histamine release; Generally, insignificant clinically but may occur with all the nmbas in large bolus doses, administered rapidly. Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant histamine release, producing tachycardia, hypotension, and skin flushing. The purposes of. Histamine Release With Cisatracurium.
From www.semanticscholar.org
Figure 2 from Hemodynamic effects and histamine release elicited by the Histamine Release With Cisatracurium Generally, insignificant clinically but may occur with all the nmbas in large bolus doses, administered rapidly. This activity reviews indications, mechanism of action, administration, contraindications, monitoring, and toxicity associated with cisatracurium and the role of. Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant histamine release, producing tachycardia, hypotension, and skin flushing. The purposes. Histamine Release With Cisatracurium.
From www.researchgate.net
Overexpression of Chrna1 abolished the effect of cisatracurium on Histamine Release With Cisatracurium Histamine release can be minimised by administering slowly or in. Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant histamine release, producing tachycardia, hypotension, and skin flushing. Generally, insignificant clinically but may occur with all the nmbas in large bolus doses, administered rapidly. The purposes of our study were to compare equipotent doses of. Histamine Release With Cisatracurium.
From www.researchgate.net
Central role of histamine in druginduced acute myocardial infarction Histamine Release With Cisatracurium The purposes of our study were to compare equipotent doses of vecuronium and cisatracurium for chemical, systemic, or. Intubating doses of atracurium (0.5 mg/kg or 2 × ed 95) can cause clinically relevant histamine release, producing tachycardia, hypotension, and skin flushing. Cardiovascular effects are associated with significant histamine release; Generally, insignificant clinically but may occur with all the nmbas in. Histamine Release With Cisatracurium.