Ice Table For Dissociation Of Acetic Acid . This can be represented by subtracting x from the original acetic acid. Using an ice table, we can find the ph of the solution when the sodium acetate is added: These tables are useful for working out concentrations of reactants and products in equilibrium. This calculator can be used to fill in values of an ice table and find the equilibrium constant of the reaction, initial concentration of reactant, and. For every acetic acid molecule that dissociates, one acetate ion and one hydronium ion is produced. Ice stands for initial, change, equilibrium. Initially, the ph of a weak acid like. Crc handbook of chemistry and physics, 84th edition (2004). In titrating a weak acid with a strong base, different calculation methods are applied at various stages. There is initially a 0.50m concentration of acetic acid. 46 rows source of data:
from www.chegg.com
Crc handbook of chemistry and physics, 84th edition (2004). Initially, the ph of a weak acid like. This calculator can be used to fill in values of an ice table and find the equilibrium constant of the reaction, initial concentration of reactant, and. There is initially a 0.50m concentration of acetic acid. 46 rows source of data: This can be represented by subtracting x from the original acetic acid. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. These tables are useful for working out concentrations of reactants and products in equilibrium. For every acetic acid molecule that dissociates, one acetate ion and one hydronium ion is produced. Ice stands for initial, change, equilibrium.
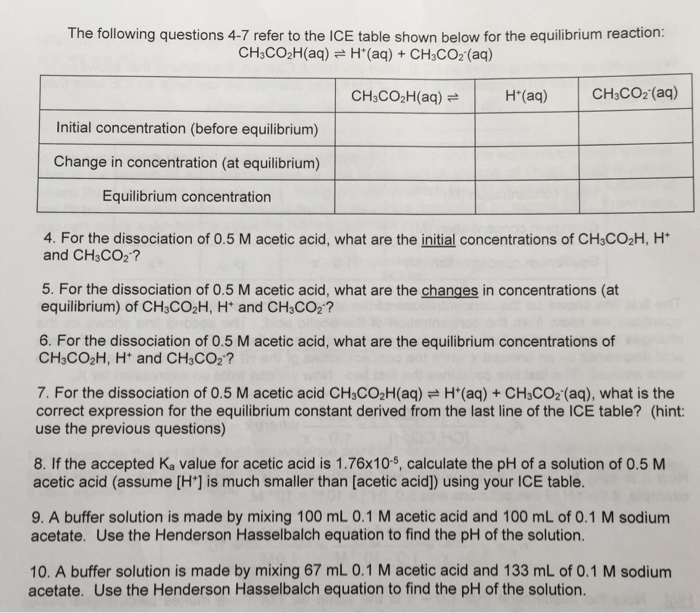
Solved The following questions 47 refer to the ICE table
Ice Table For Dissociation Of Acetic Acid This calculator can be used to fill in values of an ice table and find the equilibrium constant of the reaction, initial concentration of reactant, and. This calculator can be used to fill in values of an ice table and find the equilibrium constant of the reaction, initial concentration of reactant, and. Initially, the ph of a weak acid like. Ice stands for initial, change, equilibrium. These tables are useful for working out concentrations of reactants and products in equilibrium. Using an ice table, we can find the ph of the solution when the sodium acetate is added: For every acetic acid molecule that dissociates, one acetate ion and one hydronium ion is produced. There is initially a 0.50m concentration of acetic acid. Crc handbook of chemistry and physics, 84th edition (2004). This can be represented by subtracting x from the original acetic acid. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. 46 rows source of data:
From www.youtube.com
General Chemistry III Solving ICETables for Diprotic Systems YouTube Ice Table For Dissociation Of Acetic Acid For every acetic acid molecule that dissociates, one acetate ion and one hydronium ion is produced. Crc handbook of chemistry and physics, 84th edition (2004). This can be represented by subtracting x from the original acetic acid. Using an ice table, we can find the ph of the solution when the sodium acetate is added: Initially, the ph of a. Ice Table For Dissociation Of Acetic Acid.
From trenahaziim.blogspot.com
13+ Equilibrium Calculations With Ice Tables Worksheet TrenaHaziim Ice Table For Dissociation Of Acetic Acid This can be represented by subtracting x from the original acetic acid. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. Ice stands for initial, change, equilibrium. There is initially a 0.50m concentration of acetic acid. This calculator can be used to fill in values of an ice table and find the. Ice Table For Dissociation Of Acetic Acid.
From www.chegg.com
Solved Find Ka with ICE table for acetic acid and acetic Ice Table For Dissociation Of Acetic Acid These tables are useful for working out concentrations of reactants and products in equilibrium. For every acetic acid molecule that dissociates, one acetate ion and one hydronium ion is produced. This can be represented by subtracting x from the original acetic acid. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. Crc. Ice Table For Dissociation Of Acetic Acid.
From www.chegg.com
Solved 7. Write a balanced equation for the dissociation Ice Table For Dissociation Of Acetic Acid Crc handbook of chemistry and physics, 84th edition (2004). 46 rows source of data: These tables are useful for working out concentrations of reactants and products in equilibrium. Ice stands for initial, change, equilibrium. This calculator can be used to fill in values of an ice table and find the equilibrium constant of the reaction, initial concentration of reactant, and.. Ice Table For Dissociation Of Acetic Acid.
From www.bharatagritech.com
To Chem The Conductivity Of Mol Per Liter Acetic, 51 OFF Ice Table For Dissociation Of Acetic Acid 46 rows source of data: Using an ice table, we can find the ph of the solution when the sodium acetate is added: Crc handbook of chemistry and physics, 84th edition (2004). In titrating a weak acid with a strong base, different calculation methods are applied at various stages. For every acetic acid molecule that dissociates, one acetate ion and. Ice Table For Dissociation Of Acetic Acid.
From general.chemistrysteps.com
pH of a Weak Acid Chemistry Steps Ice Table For Dissociation Of Acetic Acid 46 rows source of data: In titrating a weak acid with a strong base, different calculation methods are applied at various stages. Using an ice table, we can find the ph of the solution when the sodium acetate is added: Initially, the ph of a weak acid like. Ice stands for initial, change, equilibrium. These tables are useful for working. Ice Table For Dissociation Of Acetic Acid.
From www.numerade.com
SOLVED In the ICE table for the dissociation of acetic acid, what is Ice Table For Dissociation Of Acetic Acid This can be represented by subtracting x from the original acetic acid. 46 rows source of data: This calculator can be used to fill in values of an ice table and find the equilibrium constant of the reaction, initial concentration of reactant, and. Ice stands for initial, change, equilibrium. Crc handbook of chemistry and physics, 84th edition (2004). These tables. Ice Table For Dissociation Of Acetic Acid.
From www.youtube.com
Chemistry Acid Dissociation Reactions using ICE Table YouTube Ice Table For Dissociation Of Acetic Acid In titrating a weak acid with a strong base, different calculation methods are applied at various stages. This can be represented by subtracting x from the original acetic acid. For every acetic acid molecule that dissociates, one acetate ion and one hydronium ion is produced. There is initially a 0.50m concentration of acetic acid. This calculator can be used to. Ice Table For Dissociation Of Acetic Acid.
From www.bartleby.com
Answered 2.) Answer the following questions… bartleby Ice Table For Dissociation Of Acetic Acid 46 rows source of data: This calculator can be used to fill in values of an ice table and find the equilibrium constant of the reaction, initial concentration of reactant, and. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. Using an ice table, we can find the ph of the solution. Ice Table For Dissociation Of Acetic Acid.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID6776086 Ice Table For Dissociation Of Acetic Acid This can be represented by subtracting x from the original acetic acid. Crc handbook of chemistry and physics, 84th edition (2004). In titrating a weak acid with a strong base, different calculation methods are applied at various stages. Ice stands for initial, change, equilibrium. These tables are useful for working out concentrations of reactants and products in equilibrium. Using an. Ice Table For Dissociation Of Acetic Acid.
From www.chegg.com
Solved CH3COOH + H2O → CH3COO + H3O+ 8. In Activity 2, Ice Table For Dissociation Of Acetic Acid There is initially a 0.50m concentration of acetic acid. For every acetic acid molecule that dissociates, one acetate ion and one hydronium ion is produced. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. 46 rows source of data: Crc handbook of chemistry and physics, 84th edition (2004). Using an ice table,. Ice Table For Dissociation Of Acetic Acid.
From www.youtube.com
Chemical Equilibrium V The ICE Table Introduction YouTube Ice Table For Dissociation Of Acetic Acid Using an ice table, we can find the ph of the solution when the sodium acetate is added: For every acetic acid molecule that dissociates, one acetate ion and one hydronium ion is produced. 46 rows source of data: In titrating a weak acid with a strong base, different calculation methods are applied at various stages. There is initially a. Ice Table For Dissociation Of Acetic Acid.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download Ice Table For Dissociation Of Acetic Acid In titrating a weak acid with a strong base, different calculation methods are applied at various stages. These tables are useful for working out concentrations of reactants and products in equilibrium. This calculator can be used to fill in values of an ice table and find the equilibrium constant of the reaction, initial concentration of reactant, and. For every acetic. Ice Table For Dissociation Of Acetic Acid.
From general.chemistrysteps.com
The Common Ion Effect Chemistry Steps Ice Table For Dissociation Of Acetic Acid These tables are useful for working out concentrations of reactants and products in equilibrium. Crc handbook of chemistry and physics, 84th edition (2004). Using an ice table, we can find the ph of the solution when the sodium acetate is added: In titrating a weak acid with a strong base, different calculation methods are applied at various stages. For every. Ice Table For Dissociation Of Acetic Acid.
From www.numerade.com
SOLVEDThe dissociation of acetic acid, CH3COOH, has an equilibrium Ice Table For Dissociation Of Acetic Acid Crc handbook of chemistry and physics, 84th edition (2004). This calculator can be used to fill in values of an ice table and find the equilibrium constant of the reaction, initial concentration of reactant, and. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. Ice stands for initial, change, equilibrium. For every. Ice Table For Dissociation Of Acetic Acid.
From www.chegg.com
Solved The following questions 47 refer to the ICE table Ice Table For Dissociation Of Acetic Acid Crc handbook of chemistry and physics, 84th edition (2004). 46 rows source of data: For every acetic acid molecule that dissociates, one acetate ion and one hydronium ion is produced. This calculator can be used to fill in values of an ice table and find the equilibrium constant of the reaction, initial concentration of reactant, and. In titrating a weak. Ice Table For Dissociation Of Acetic Acid.
From www.youtube.com
Ka, Kb, pH, pOH, H3O+ and OH Calculations ICE Tables (Acids and Bases Ice Table For Dissociation Of Acetic Acid Ice stands for initial, change, equilibrium. There is initially a 0.50m concentration of acetic acid. Using an ice table, we can find the ph of the solution when the sodium acetate is added: This can be represented by subtracting x from the original acetic acid. This calculator can be used to fill in values of an ice table and find. Ice Table For Dissociation Of Acetic Acid.
From www.chegg.com
Solved . 5. Build an ICE table for the acid dissociation of Ice Table For Dissociation Of Acetic Acid For every acetic acid molecule that dissociates, one acetate ion and one hydronium ion is produced. Ice stands for initial, change, equilibrium. Initially, the ph of a weak acid like. This calculator can be used to fill in values of an ice table and find the equilibrium constant of the reaction, initial concentration of reactant, and. These tables are useful. Ice Table For Dissociation Of Acetic Acid.
From www.chegg.com
Solved Below is the ICE table for the dissociation reaction Ice Table For Dissociation Of Acetic Acid This can be represented by subtracting x from the original acetic acid. There is initially a 0.50m concentration of acetic acid. For every acetic acid molecule that dissociates, one acetate ion and one hydronium ion is produced. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. Ice stands for initial, change, equilibrium.. Ice Table For Dissociation Of Acetic Acid.
From www.conquerhsc.com
HSC Chemistry Module 5 Inquiry Question 3 Ice Table For Dissociation Of Acetic Acid Crc handbook of chemistry and physics, 84th edition (2004). 46 rows source of data: This calculator can be used to fill in values of an ice table and find the equilibrium constant of the reaction, initial concentration of reactant, and. This can be represented by subtracting x from the original acetic acid. Using an ice table, we can find the. Ice Table For Dissociation Of Acetic Acid.
From sciencenotes.org
What Is Glacial Acetic Acid? Ice Table For Dissociation Of Acetic Acid 46 rows source of data: There is initially a 0.50m concentration of acetic acid. This calculator can be used to fill in values of an ice table and find the equilibrium constant of the reaction, initial concentration of reactant, and. Crc handbook of chemistry and physics, 84th edition (2004). For every acetic acid molecule that dissociates, one acetate ion and. Ice Table For Dissociation Of Acetic Acid.
From www.semanticscholar.org
[PDF] Acetic acid—water system thermodynamical correlation of vapor Ice Table For Dissociation Of Acetic Acid This calculator can be used to fill in values of an ice table and find the equilibrium constant of the reaction, initial concentration of reactant, and. There is initially a 0.50m concentration of acetic acid. 46 rows source of data: This can be represented by subtracting x from the original acetic acid. In titrating a weak acid with a strong. Ice Table For Dissociation Of Acetic Acid.
From www.sliderbase.com
Acids, pH and Equilibrium Presentation Chemistry Ice Table For Dissociation Of Acetic Acid These tables are useful for working out concentrations of reactants and products in equilibrium. Crc handbook of chemistry and physics, 84th edition (2004). Ice stands for initial, change, equilibrium. This calculator can be used to fill in values of an ice table and find the equilibrium constant of the reaction, initial concentration of reactant, and. Using an ice table, we. Ice Table For Dissociation Of Acetic Acid.
From www.tessshebaylo.com
Dissociation Of Ethanoic Acid In Water Equation Tessshebaylo Ice Table For Dissociation Of Acetic Acid In titrating a weak acid with a strong base, different calculation methods are applied at various stages. This calculator can be used to fill in values of an ice table and find the equilibrium constant of the reaction, initial concentration of reactant, and. Initially, the ph of a weak acid like. Crc handbook of chemistry and physics, 84th edition (2004).. Ice Table For Dissociation Of Acetic Acid.
From www.tessshebaylo.com
Dissociation Of Acetic Acid In Water Equation Tessshebaylo Ice Table For Dissociation Of Acetic Acid These tables are useful for working out concentrations of reactants and products in equilibrium. Ice stands for initial, change, equilibrium. This calculator can be used to fill in values of an ice table and find the equilibrium constant of the reaction, initial concentration of reactant, and. 46 rows source of data: Crc handbook of chemistry and physics, 84th edition (2004).. Ice Table For Dissociation Of Acetic Acid.
From mavink.com
Acid Dissociation Constant Table Ice Table For Dissociation Of Acetic Acid Initially, the ph of a weak acid like. This can be represented by subtracting x from the original acetic acid. Ice stands for initial, change, equilibrium. These tables are useful for working out concentrations of reactants and products in equilibrium. This calculator can be used to fill in values of an ice table and find the equilibrium constant of the. Ice Table For Dissociation Of Acetic Acid.
From www.youtube.com
DAT Finding Percent Dissociation of a Weak Acid (Acetic Acid) YouTube Ice Table For Dissociation Of Acetic Acid Initially, the ph of a weak acid like. Using an ice table, we can find the ph of the solution when the sodium acetate is added: For every acetic acid molecule that dissociates, one acetate ion and one hydronium ion is produced. This can be represented by subtracting x from the original acetic acid. These tables are useful for working. Ice Table For Dissociation Of Acetic Acid.
From www.researchgate.net
DISSOCIATION AND METAL COMPLEX EQUILIBRIUM (FORMATION) CONSTANTS FOR Ice Table For Dissociation Of Acetic Acid For every acetic acid molecule that dissociates, one acetate ion and one hydronium ion is produced. Ice stands for initial, change, equilibrium. This can be represented by subtracting x from the original acetic acid. 46 rows source of data: Using an ice table, we can find the ph of the solution when the sodium acetate is added: Crc handbook of. Ice Table For Dissociation Of Acetic Acid.
From www.tessshebaylo.com
Ionization Of Acetic Acid Equilibrium Equation Tessshebaylo Ice Table For Dissociation Of Acetic Acid Crc handbook of chemistry and physics, 84th edition (2004). Ice stands for initial, change, equilibrium. This calculator can be used to fill in values of an ice table and find the equilibrium constant of the reaction, initial concentration of reactant, and. Using an ice table, we can find the ph of the solution when the sodium acetate is added: 46. Ice Table For Dissociation Of Acetic Acid.
From www.sliderbase.com
Acids, pH and Equilibrium Presentation Chemistry Ice Table For Dissociation Of Acetic Acid In titrating a weak acid with a strong base, different calculation methods are applied at various stages. Initially, the ph of a weak acid like. There is initially a 0.50m concentration of acetic acid. For every acetic acid molecule that dissociates, one acetate ion and one hydronium ion is produced. 46 rows source of data: This calculator can be used. Ice Table For Dissociation Of Acetic Acid.
From www.semanticscholar.org
Table 1 from Determination of Dissociation Constants of Malonic Acid in Ice Table For Dissociation Of Acetic Acid This calculator can be used to fill in values of an ice table and find the equilibrium constant of the reaction, initial concentration of reactant, and. These tables are useful for working out concentrations of reactants and products in equilibrium. Initially, the ph of a weak acid like. For every acetic acid molecule that dissociates, one acetate ion and one. Ice Table For Dissociation Of Acetic Acid.
From oneclass.com
OneClass Determine the equilibrium concentrations for the reaction of Ice Table For Dissociation Of Acetic Acid Ice stands for initial, change, equilibrium. There is initially a 0.50m concentration of acetic acid. These tables are useful for working out concentrations of reactants and products in equilibrium. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. For every acetic acid molecule that dissociates, one acetate ion and one hydronium ion. Ice Table For Dissociation Of Acetic Acid.
From www.slideserve.com
PPT Equilibrium Acids and Bases PowerPoint Presentation, free Ice Table For Dissociation Of Acetic Acid 46 rows source of data: Ice stands for initial, change, equilibrium. Crc handbook of chemistry and physics, 84th edition (2004). This calculator can be used to fill in values of an ice table and find the equilibrium constant of the reaction, initial concentration of reactant, and. These tables are useful for working out concentrations of reactants and products in equilibrium.. Ice Table For Dissociation Of Acetic Acid.
From goodttorials.blogspot.com
How To Find Equilibrium Constant Using Ice Table Ice Table For Dissociation Of Acetic Acid This calculator can be used to fill in values of an ice table and find the equilibrium constant of the reaction, initial concentration of reactant, and. This can be represented by subtracting x from the original acetic acid. There is initially a 0.50m concentration of acetic acid. 46 rows source of data: These tables are useful for working out concentrations. Ice Table For Dissociation Of Acetic Acid.
From www.tes.com
CALCULATING Ka Kb Kw, ICE Tables, ACID DISSOCIATION CONSTANT Power Ice Table For Dissociation Of Acetic Acid Using an ice table, we can find the ph of the solution when the sodium acetate is added: These tables are useful for working out concentrations of reactants and products in equilibrium. Initially, the ph of a weak acid like. This calculator can be used to fill in values of an ice table and find the equilibrium constant of the. Ice Table For Dissociation Of Acetic Acid.