Copper 2 Oxide + Nitric Acid . concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide (no 2) gas and water as products. copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2 (aq) + 2no 2 (g). The second part comes from the. Copper + nitric acid = copper (ii) nitrate + nitric oxide (radical) + water. copper(ii) oxide + nitric acid = copper + nitrate radical + water. copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. A black solid, it is one of the two stable oxides of copper,. for example, copper does not react with dilute acids, so copper salts are made using copper oxide or copper carbonate, not copper metal. the name of a salt has two parts. The first part comes from the metal, metal oxide or metal carbonate. Copper (ii) oxide + nitric acid = copper (ii) oxide + dinitrogen + water. One mole of copper(ii) oxide [cuo] and two moles of nitric acid [hno 3]. One mole of copper (ii) oxide [cuo] and zero. In this reaction, copper is oxidized.
from www.youtube.com
One mole of copper(ii) oxide [cuo] and two moles of nitric acid [hno 3]. Copper + nitric acid = copper (ii) nitrate + nitric oxide (radical) + water. A black solid, it is one of the two stable oxides of copper,. The first part comes from the metal, metal oxide or metal carbonate. copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. In this reaction, copper is oxidized. One mole of copper (ii) oxide [cuo] and zero. for example, copper does not react with dilute acids, so copper salts are made using copper oxide or copper carbonate, not copper metal. Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2 (aq) + 2no 2 (g). the name of a salt has two parts.
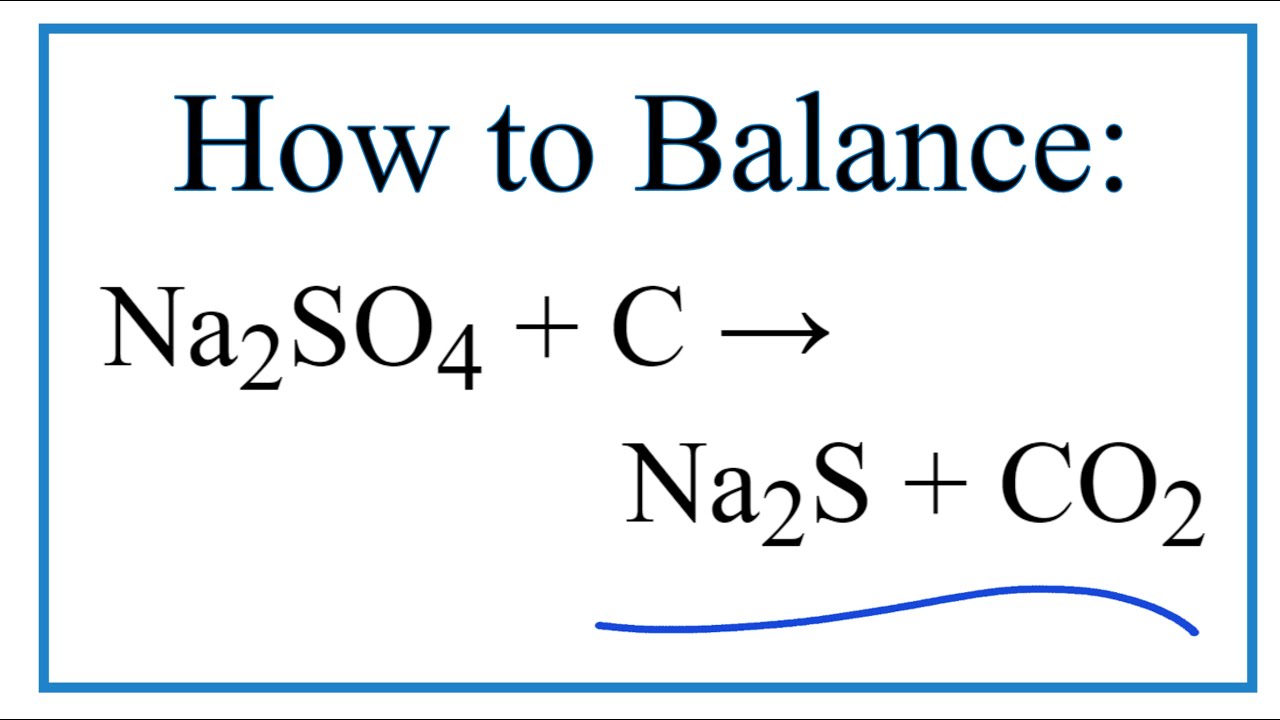
How to Balance Na2SO4 + C = Na2S + CO2 YouTube
Copper 2 Oxide + Nitric Acid copper(ii) oxide + nitric acid = copper + nitrate radical + water. One mole of copper (ii) oxide [cuo] and zero. The second part comes from the. concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide (no 2) gas and water as products. copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2 (aq) + 2no 2 (g). for example, copper does not react with dilute acids, so copper salts are made using copper oxide or copper carbonate, not copper metal. The first part comes from the metal, metal oxide or metal carbonate. copper(ii) oxide + nitric acid = copper + nitrate radical + water. In this reaction, copper is oxidized. copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. One mole of copper(ii) oxide [cuo] and two moles of nitric acid [hno 3]. Copper (ii) oxide + nitric acid = copper (ii) oxide + dinitrogen + water. A black solid, it is one of the two stable oxides of copper,. Copper + nitric acid = copper (ii) nitrate + nitric oxide (radical) + water. the name of a salt has two parts.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper 2 Oxide + Nitric Acid concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide (no 2) gas and water as products. One mole of copper(ii) oxide [cuo] and two moles of nitric acid [hno 3]. The second part comes from the. the name of a salt has two parts. One mole of copper (ii) oxide [cuo]. Copper 2 Oxide + Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper 2 Oxide + Nitric Acid Copper + nitric acid = copper (ii) nitrate + nitric oxide (radical) + water. copper(ii) oxide + nitric acid = copper + nitrate radical + water. copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. One mole of copper (ii) oxide [cuo] and zero. A black solid, it is one of the two. Copper 2 Oxide + Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper 2 Oxide + Nitric Acid Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2 (aq) + 2no 2 (g). Copper (ii) oxide + nitric acid = copper (ii) oxide + dinitrogen + water. One mole of copper(ii) oxide [cuo] and two moles of nitric acid [hno 3]. concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide. Copper 2 Oxide + Nitric Acid.
From www.youtube.com
Copper plus Nitric Acid Animation YouTube Copper 2 Oxide + Nitric Acid The first part comes from the metal, metal oxide or metal carbonate. The second part comes from the. One mole of copper(ii) oxide [cuo] and two moles of nitric acid [hno 3]. concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide (no 2) gas and water as products. A black solid, it. Copper 2 Oxide + Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper 2 Oxide + Nitric Acid Copper + nitric acid = copper (ii) nitrate + nitric oxide (radical) + water. One mole of copper(ii) oxide [cuo] and two moles of nitric acid [hno 3]. copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. Copper (ii) oxide + nitric acid = copper (ii) oxide + dinitrogen + water. One mole of. Copper 2 Oxide + Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper 2 Oxide + Nitric Acid copper(ii) oxide + nitric acid = copper + nitrate radical + water. copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. Copper + nitric acid = copper (ii) nitrate + nitric oxide (radical) + water. concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide (no 2). Copper 2 Oxide + Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper 2 Oxide + Nitric Acid copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. the name of a salt has two parts. A black solid, it is one of the two stable oxides of copper,. for example, copper does not react with dilute acids, so copper salts are made using copper oxide or copper carbonate, not copper metal.. Copper 2 Oxide + Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper 2 Oxide + Nitric Acid for example, copper does not react with dilute acids, so copper salts are made using copper oxide or copper carbonate, not copper metal. copper(ii) oxide + nitric acid = copper + nitrate radical + water. copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. copper reacts with nitric acid, forming aqueous copper. Copper 2 Oxide + Nitric Acid.
From www.numerade.com
SOLVED The reaction between solid copper and nitric acid to form Copper 2 Oxide + Nitric Acid One mole of copper (ii) oxide [cuo] and zero. Copper (ii) oxide + nitric acid = copper (ii) oxide + dinitrogen + water. Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2 (aq) + 2no 2 (g). for example, copper does not react with dilute acids, so copper salts are made using copper oxide or copper carbonate, not. Copper 2 Oxide + Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper 2 Oxide + Nitric Acid The first part comes from the metal, metal oxide or metal carbonate. One mole of copper (ii) oxide [cuo] and zero. copper(ii) oxide + nitric acid = copper + nitrate radical + water. the name of a salt has two parts. copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. One mole. Copper 2 Oxide + Nitric Acid.
From www.chegg.com
Solved Step 1. Reaction of Copper with Nitric Acid (2 Copper 2 Oxide + Nitric Acid In this reaction, copper is oxidized. One mole of copper (ii) oxide [cuo] and zero. The first part comes from the metal, metal oxide or metal carbonate. copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. A black solid, it is one of the two stable oxides of copper,. Copper + nitric acid = copper. Copper 2 Oxide + Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper 2 Oxide + Nitric Acid In this reaction, copper is oxidized. One mole of copper (ii) oxide [cuo] and zero. The first part comes from the metal, metal oxide or metal carbonate. copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. Copper (ii) oxide + nitric acid = copper (ii) oxide + dinitrogen + water. the name of a. Copper 2 Oxide + Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper 2 Oxide + Nitric Acid The first part comes from the metal, metal oxide or metal carbonate. The second part comes from the. concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide (no 2) gas and water as products. Copper + nitric acid = copper (ii) nitrate + nitric oxide (radical) + water. the name of. Copper 2 Oxide + Nitric Acid.
From www.sciencephoto.com
Copper Reacts with Nitric Acid Stock Image C028/1266 Science Copper 2 Oxide + Nitric Acid Copper + nitric acid = copper (ii) nitrate + nitric oxide (radical) + water. copper(ii) oxide + nitric acid = copper + nitrate radical + water. copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. for example, copper. Copper 2 Oxide + Nitric Acid.
From onlinelibrary.wiley.com
Nitrite and Nitric Oxide Interconversion at Mononuclear Copper(II Copper 2 Oxide + Nitric Acid In this reaction, copper is oxidized. One mole of copper (ii) oxide [cuo] and zero. copper(ii) oxide + nitric acid = copper + nitrate radical + water. Copper + nitric acid = copper (ii) nitrate + nitric oxide (radical) + water. copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. Copper (ii) oxide +. Copper 2 Oxide + Nitric Acid.
From blog.thepipingmart.com
What Happens When Copper is Heated with Concentrated Nitric Acid? Copper 2 Oxide + Nitric Acid One mole of copper (ii) oxide [cuo] and zero. Copper + nitric acid = copper (ii) nitrate + nitric oxide (radical) + water. copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. for example, copper does not react with dilute acids, so copper salts are made using copper oxide or copper carbonate, not. Copper 2 Oxide + Nitric Acid.
From www.youtube.com
Copper and Nitric acid reaction YouTube Copper 2 Oxide + Nitric Acid concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide (no 2) gas and water as products. One mole of copper (ii) oxide [cuo] and zero. Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2 (aq) + 2no 2 (g). copper(ii) oxide or cupric oxide is an inorganic compound with the. Copper 2 Oxide + Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper 2 Oxide + Nitric Acid One mole of copper(ii) oxide [cuo] and two moles of nitric acid [hno 3]. for example, copper does not react with dilute acids, so copper salts are made using copper oxide or copper carbonate, not copper metal. The second part comes from the. A black solid, it is one of the two stable oxides of copper,. Cu(s) + 4hno. Copper 2 Oxide + Nitric Acid.
From byjus.com
Copper reacts with dilute nitric acid and forms copper nitrate, nitric Copper 2 Oxide + Nitric Acid One mole of copper(ii) oxide [cuo] and two moles of nitric acid [hno 3]. In this reaction, copper is oxidized. The first part comes from the metal, metal oxide or metal carbonate. copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. copper(ii) oxide + nitric acid = copper + nitrate radical + water.. Copper 2 Oxide + Nitric Acid.
From www.numerade.com
SOLVED 2 REACTION I Dissolution of copper metal with nitric acid Copper 2 Oxide + Nitric Acid One mole of copper(ii) oxide [cuo] and two moles of nitric acid [hno 3]. concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide (no 2) gas and water as products. for example, copper does not react with dilute acids, so copper salts are made using copper oxide or copper carbonate, not. Copper 2 Oxide + Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper 2 Oxide + Nitric Acid One mole of copper(ii) oxide [cuo] and two moles of nitric acid [hno 3]. copper(ii) oxide + nitric acid = copper + nitrate radical + water. A black solid, it is one of the two stable oxides of copper,. concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide (no 2) gas. Copper 2 Oxide + Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper 2 Oxide + Nitric Acid In this reaction, copper is oxidized. copper(ii) oxide + nitric acid = copper + nitrate radical + water. copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. Copper (ii) oxide + nitric acid = copper (ii) oxide + dinitrogen + water. Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2 (aq) + 2no. Copper 2 Oxide + Nitric Acid.
From sciencenotes.org
Copper and Nitric Acid Chemistry Demonstration Copper 2 Oxide + Nitric Acid concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide (no 2) gas and water as products. the name of a salt has two parts. copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. One mole of copper (ii) oxide [cuo] and zero. Copper + nitric acid. Copper 2 Oxide + Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper 2 Oxide + Nitric Acid One mole of copper(ii) oxide [cuo] and two moles of nitric acid [hno 3]. The first part comes from the metal, metal oxide or metal carbonate. copper(ii) oxide + nitric acid = copper + nitrate radical + water. A black solid, it is one of the two stable oxides of copper,. One mole of copper (ii) oxide [cuo] and. Copper 2 Oxide + Nitric Acid.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Copper 2 Oxide + Nitric Acid copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. the name of a salt has two parts. Copper + nitric acid = copper (ii) nitrate + nitric oxide (radical) + water. A black solid, it is one of the two stable oxides of copper,. One mole of copper (ii) oxide [cuo] and zero.. Copper 2 Oxide + Nitric Acid.
From edu-rsc-org-s.webvpn.bjmu.doc110.com
Finding the formula of copper(II) oxide Experiment RSC Education Copper 2 Oxide + Nitric Acid for example, copper does not react with dilute acids, so copper salts are made using copper oxide or copper carbonate, not copper metal. One mole of copper (ii) oxide [cuo] and zero. The second part comes from the. A black solid, it is one of the two stable oxides of copper,. Copper (ii) oxide + nitric acid = copper. Copper 2 Oxide + Nitric Acid.
From www.alamy.com
Concentrated Nitric Acid Reacting with Copper and Generating Nitrogen Copper 2 Oxide + Nitric Acid A black solid, it is one of the two stable oxides of copper,. for example, copper does not react with dilute acids, so copper salts are made using copper oxide or copper carbonate, not copper metal. copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. copper(ii) oxide + nitric acid = copper. Copper 2 Oxide + Nitric Acid.
From www.youtube.com
Cu+HNO3=Cu(NO3)2+NO2+H2O Balanced EquationCopper+Nitric acid=Copper Copper 2 Oxide + Nitric Acid Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2 (aq) + 2no 2 (g). copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide (no 2) gas and water as products. One mole of copper (ii) oxide [cuo]. Copper 2 Oxide + Nitric Acid.
From www.chegg.com
Solved What is the net ionic equation for copper(II) Copper 2 Oxide + Nitric Acid A black solid, it is one of the two stable oxides of copper,. The second part comes from the. One mole of copper(ii) oxide [cuo] and two moles of nitric acid [hno 3]. copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. the name of a salt has two parts. The first part. Copper 2 Oxide + Nitric Acid.
From www.slideserve.com
PPT Chemical Reactions Copper Reactions PowerPoint Presentation Copper 2 Oxide + Nitric Acid Copper + nitric acid = copper (ii) nitrate + nitric oxide (radical) + water. One mole of copper(ii) oxide [cuo] and two moles of nitric acid [hno 3]. A black solid, it is one of the two stable oxides of copper,. for example, copper does not react with dilute acids, so copper salts are made using copper oxide or. Copper 2 Oxide + Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper 2 Oxide + Nitric Acid the name of a salt has two parts. Copper (ii) oxide + nitric acid = copper (ii) oxide + dinitrogen + water. copper(ii) oxide + nitric acid = copper + nitrate radical + water. The first part comes from the metal, metal oxide or metal carbonate. for example, copper does not react with dilute acids, so copper. Copper 2 Oxide + Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper 2 Oxide + Nitric Acid Copper + nitric acid = copper (ii) nitrate + nitric oxide (radical) + water. The first part comes from the metal, metal oxide or metal carbonate. In this reaction, copper is oxidized. Copper (ii) oxide + nitric acid = copper (ii) oxide + dinitrogen + water. The second part comes from the. One mole of copper(ii) oxide [cuo] and two. Copper 2 Oxide + Nitric Acid.
From www.youtube.com
How to Balance Na2SO4 + C = Na2S + CO2 YouTube Copper 2 Oxide + Nitric Acid for example, copper does not react with dilute acids, so copper salts are made using copper oxide or copper carbonate, not copper metal. Copper + nitric acid = copper (ii) nitrate + nitric oxide (radical) + water. concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide (no 2) gas and water. Copper 2 Oxide + Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper 2 Oxide + Nitric Acid A black solid, it is one of the two stable oxides of copper,. One mole of copper (ii) oxide [cuo] and zero. Copper (ii) oxide + nitric acid = copper (ii) oxide + dinitrogen + water. copper(ii) oxide + nitric acid = copper + nitrate radical + water. copper(ii) oxide or cupric oxide is an inorganic compound with. Copper 2 Oxide + Nitric Acid.
From sciencenotes.org
Copper and Nitric Acid Chemistry Demonstration Copper 2 Oxide + Nitric Acid Copper + nitric acid = copper (ii) nitrate + nitric oxide (radical) + water. copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. A black solid, it is one of the two stable oxides of copper,. Copper (ii) oxide + nitric acid = copper (ii) oxide + dinitrogen + water. The first part comes. Copper 2 Oxide + Nitric Acid.