How To Analyze Clinical Trial Data . Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. What is a clinical trial? This article reviews key portions of the process of applying research results to clinical practice. There are two types of data analyses of randomized clinical trials (rcts). In publications of clinical trials, the first table in the paper usually summarizes the characteristics of the study sample, and readers should. The first step involves defining the study population. Trials should adhere to the consort statement. Clinicians must be able to critically appraise clinical trials to determine their internal validity. A clinical trial is any research study that prospectively assigns human participants or groups of humans to one or. Assuming that a clinical trial will produce data.
from www.slideteam.net
There are two types of data analyses of randomized clinical trials (rcts). Assuming that a clinical trial will produce data. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. This article reviews key portions of the process of applying research results to clinical practice. A clinical trial is any research study that prospectively assigns human participants or groups of humans to one or. In publications of clinical trials, the first table in the paper usually summarizes the characteristics of the study sample, and readers should. What is a clinical trial? The first step involves defining the study population. Trials should adhere to the consort statement. Clinicians must be able to critically appraise clinical trials to determine their internal validity.
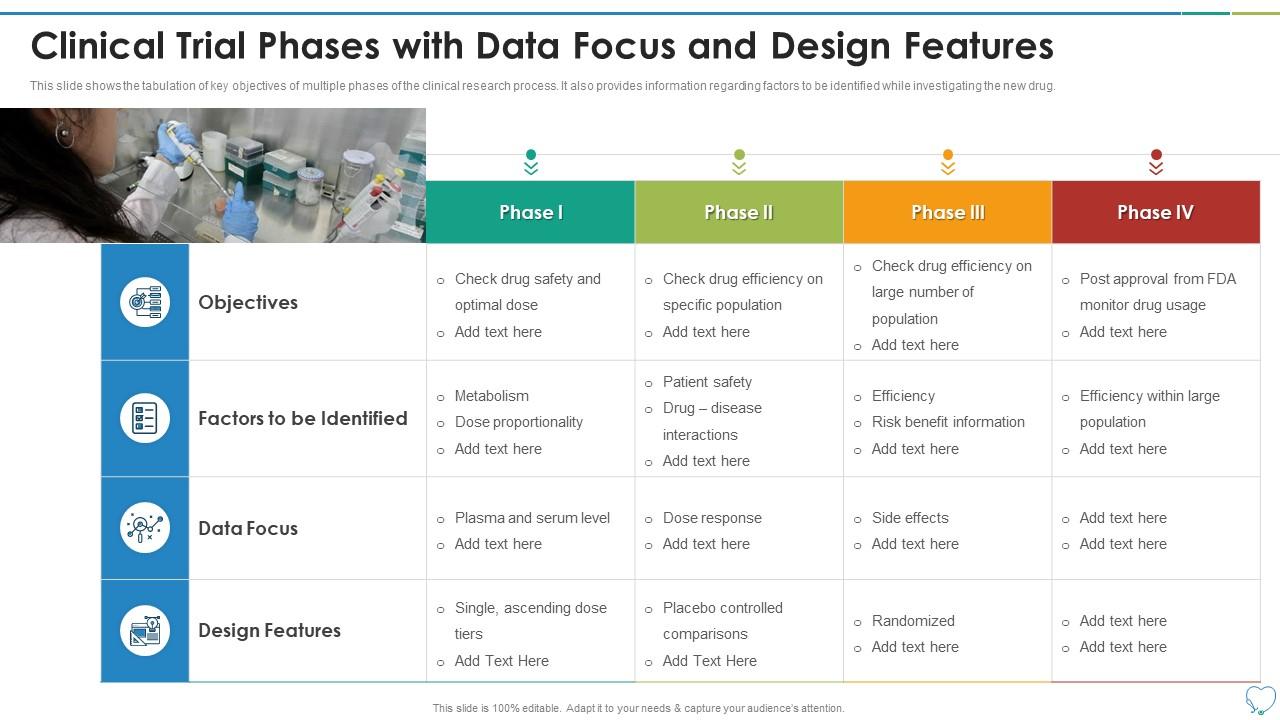
Clinical Trial Phases Data Focus And Design Features Presentation
How To Analyze Clinical Trial Data Clinicians must be able to critically appraise clinical trials to determine their internal validity. This article reviews key portions of the process of applying research results to clinical practice. In publications of clinical trials, the first table in the paper usually summarizes the characteristics of the study sample, and readers should. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. Clinicians must be able to critically appraise clinical trials to determine their internal validity. There are two types of data analyses of randomized clinical trials (rcts). Assuming that a clinical trial will produce data. Trials should adhere to the consort statement. A clinical trial is any research study that prospectively assigns human participants or groups of humans to one or. What is a clinical trial? The first step involves defining the study population.
From www.biopharmaservices.com
Clinical Trial Data Management Services BioPharma Services How To Analyze Clinical Trial Data Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. What is a clinical trial? Trials should adhere to the consort statement. A clinical trial is any research study that prospectively assigns human participants or groups of humans to one or. This article reviews key portions of the process of applying research results to clinical. How To Analyze Clinical Trial Data.
From www.hemophilia.org
Understanding Clinical Trials NBDF How To Analyze Clinical Trial Data In publications of clinical trials, the first table in the paper usually summarizes the characteristics of the study sample, and readers should. This article reviews key portions of the process of applying research results to clinical practice. What is a clinical trial? A clinical trial is any research study that prospectively assigns human participants or groups of humans to one. How To Analyze Clinical Trial Data.
From miaspharma.com
EMA starts a pilot study to analyze clinical trial raw data MIAS Pharma How To Analyze Clinical Trial Data What is a clinical trial? A clinical trial is any research study that prospectively assigns human participants or groups of humans to one or. Trials should adhere to the consort statement. There are two types of data analyses of randomized clinical trials (rcts). Assuming that a clinical trial will produce data. This article reviews key portions of the process of. How To Analyze Clinical Trial Data.
From www.cronj.com
How Clinical Trial Management Software Leads to Faster FDA Approvals? How To Analyze Clinical Trial Data In publications of clinical trials, the first table in the paper usually summarizes the characteristics of the study sample, and readers should. A clinical trial is any research study that prospectively assigns human participants or groups of humans to one or. There are two types of data analyses of randomized clinical trials (rcts). Megan othus and us discuss complexities in. How To Analyze Clinical Trial Data.
From bmjopen.bmj.com
Sharing and reuse of individual participant data from clinical trials How To Analyze Clinical Trial Data The first step involves defining the study population. Clinicians must be able to critically appraise clinical trials to determine their internal validity. This article reviews key portions of the process of applying research results to clinical practice. What is a clinical trial? There are two types of data analyses of randomized clinical trials (rcts). A clinical trial is any research. How To Analyze Clinical Trial Data.
From www.slideteam.net
Multiple Phases Of Clinical Trial With Research Design For How To Analyze Clinical Trial Data What is a clinical trial? Assuming that a clinical trial will produce data. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. This article reviews key portions of the process of applying research results to clinical practice. Trials should adhere to the consort statement. There are two types of data analyses of randomized clinical. How To Analyze Clinical Trial Data.
From www.medidata.com
The Future of Clinical Trial Data Management How To Analyze Clinical Trial Data The first step involves defining the study population. In publications of clinical trials, the first table in the paper usually summarizes the characteristics of the study sample, and readers should. Assuming that a clinical trial will produce data. Clinicians must be able to critically appraise clinical trials to determine their internal validity. Trials should adhere to the consort statement. A. How To Analyze Clinical Trial Data.
From www.scribd.com
A Comprehensive Guide to Analyzing Clinical Trial Data PDF How To Analyze Clinical Trial Data Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. This article reviews key portions of the process of applying research results to clinical practice. There are two types of data analyses of randomized clinical trials (rcts). Trials should adhere to the consort statement. A clinical trial is any research study that prospectively assigns human. How To Analyze Clinical Trial Data.
From www.smartsheet.com
All about Clinical Trial Data Management Smartsheet How To Analyze Clinical Trial Data Assuming that a clinical trial will produce data. This article reviews key portions of the process of applying research results to clinical practice. There are two types of data analyses of randomized clinical trials (rcts). Trials should adhere to the consort statement. The first step involves defining the study population. A clinical trial is any research study that prospectively assigns. How To Analyze Clinical Trial Data.
From statisticsconsultancy.com
Clinical Trial Phases Statistical Consultancy How To Analyze Clinical Trial Data Trials should adhere to the consort statement. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. This article reviews key portions of the process of applying research results to clinical practice. There are two types of data analyses of randomized clinical trials (rcts). The first step involves defining the study population. A clinical trial. How To Analyze Clinical Trial Data.
From www.castoredc.com
Electronic Data Capture (EDC) in Clinical Trials Essential Guide How To Analyze Clinical Trial Data There are two types of data analyses of randomized clinical trials (rcts). Clinicians must be able to critically appraise clinical trials to determine their internal validity. A clinical trial is any research study that prospectively assigns human participants or groups of humans to one or. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false.. How To Analyze Clinical Trial Data.
From blogs.opentext.com
Why intelligent classification of clinical trial data is so important How To Analyze Clinical Trial Data A clinical trial is any research study that prospectively assigns human participants or groups of humans to one or. Assuming that a clinical trial will produce data. Clinicians must be able to critically appraise clinical trials to determine their internal validity. This article reviews key portions of the process of applying research results to clinical practice. The first step involves. How To Analyze Clinical Trial Data.
From www.origent.com
Clinical Trial Analysis Origent Data Sciences How To Analyze Clinical Trial Data A clinical trial is any research study that prospectively assigns human participants or groups of humans to one or. In publications of clinical trials, the first table in the paper usually summarizes the characteristics of the study sample, and readers should. There are two types of data analyses of randomized clinical trials (rcts). This article reviews key portions of the. How To Analyze Clinical Trial Data.
From www.hdruk.ac.uk
Clinical trials Day 2022 How data can make trials faster, more How To Analyze Clinical Trial Data Assuming that a clinical trial will produce data. There are two types of data analyses of randomized clinical trials (rcts). The first step involves defining the study population. In publications of clinical trials, the first table in the paper usually summarizes the characteristics of the study sample, and readers should. A clinical trial is any research study that prospectively assigns. How To Analyze Clinical Trial Data.
From clinnovo.blogspot.com
Clinnovo News Data Flow During Clinical Trial Process How To Analyze Clinical Trial Data Clinicians must be able to critically appraise clinical trials to determine their internal validity. There are two types of data analyses of randomized clinical trials (rcts). The first step involves defining the study population. Assuming that a clinical trial will produce data. Trials should adhere to the consort statement. In publications of clinical trials, the first table in the paper. How To Analyze Clinical Trial Data.
From www.altexsoft.com
Clinical Data Management Roles, Steps, and Software Tools AltexSoft How To Analyze Clinical Trial Data Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. In publications of clinical trials, the first table in the paper usually summarizes the characteristics of the study sample, and readers should. This article reviews key portions of the process of applying research results to clinical practice. A clinical trial is any research study that. How To Analyze Clinical Trial Data.
From www.slideteam.net
Clinical Trial Phases Graph Indicating Clinical Trial Phases How To Analyze Clinical Trial Data Trials should adhere to the consort statement. Assuming that a clinical trial will produce data. The first step involves defining the study population. Clinicians must be able to critically appraise clinical trials to determine their internal validity. There are two types of data analyses of randomized clinical trials (rcts). This article reviews key portions of the process of applying research. How To Analyze Clinical Trial Data.
From brasddp.com
BRAS DDP Clinical Trials Bras DDP How To Analyze Clinical Trial Data Assuming that a clinical trial will produce data. A clinical trial is any research study that prospectively assigns human participants or groups of humans to one or. In publications of clinical trials, the first table in the paper usually summarizes the characteristics of the study sample, and readers should. What is a clinical trial? This article reviews key portions of. How To Analyze Clinical Trial Data.
From www.onlinespss.com
Clinical Trial Data Analysis and Report Help How To Analyze Clinical Trial Data What is a clinical trial? The first step involves defining the study population. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. Clinicians must be able to critically appraise clinical trials to determine their internal validity. This article reviews key portions of the process of applying research results to clinical practice. Trials should adhere. How To Analyze Clinical Trial Data.
From www.slideteam.net
Clinical Trial Phases Data Focus And Design Features Presentation How To Analyze Clinical Trial Data What is a clinical trial? A clinical trial is any research study that prospectively assigns human participants or groups of humans to one or. There are two types of data analyses of randomized clinical trials (rcts). Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. Clinicians must be able to critically appraise clinical trials. How To Analyze Clinical Trial Data.
From slidesgo.com
Clinical Trial Infographics for Google Slides and PowerPoint How To Analyze Clinical Trial Data In publications of clinical trials, the first table in the paper usually summarizes the characteristics of the study sample, and readers should. This article reviews key portions of the process of applying research results to clinical practice. There are two types of data analyses of randomized clinical trials (rcts). Assuming that a clinical trial will produce data. Clinicians must be. How To Analyze Clinical Trial Data.
From slidesgo.com
Clinical Trial Infographics for Google Slides and PowerPoint How To Analyze Clinical Trial Data What is a clinical trial? A clinical trial is any research study that prospectively assigns human participants or groups of humans to one or. The first step involves defining the study population. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. In publications of clinical trials, the first table in the paper usually summarizes. How To Analyze Clinical Trial Data.
From www.jli.edu.in
Data Management in Clinical Trials JLI Blog How To Analyze Clinical Trial Data Trials should adhere to the consort statement. What is a clinical trial? A clinical trial is any research study that prospectively assigns human participants or groups of humans to one or. There are two types of data analyses of randomized clinical trials (rcts). Clinicians must be able to critically appraise clinical trials to determine their internal validity. In publications of. How To Analyze Clinical Trial Data.
From www.researchgate.net
Data flow of the clinical trial Download Scientific Diagram How To Analyze Clinical Trial Data Trials should adhere to the consort statement. In publications of clinical trials, the first table in the paper usually summarizes the characteristics of the study sample, and readers should. A clinical trial is any research study that prospectively assigns human participants or groups of humans to one or. This article reviews key portions of the process of applying research results. How To Analyze Clinical Trial Data.
From faircookbook.elixir-europe.org
7. Mapping of clinical trial data to CDISCSDTM a practical example How To Analyze Clinical Trial Data This article reviews key portions of the process of applying research results to clinical practice. In publications of clinical trials, the first table in the paper usually summarizes the characteristics of the study sample, and readers should. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. Clinicians must be able to critically appraise clinical. How To Analyze Clinical Trial Data.
From www.slideserve.com
PPT Clinical trial data PowerPoint Presentation, free download ID How To Analyze Clinical Trial Data The first step involves defining the study population. A clinical trial is any research study that prospectively assigns human participants or groups of humans to one or. What is a clinical trial? Clinicians must be able to critically appraise clinical trials to determine their internal validity. This article reviews key portions of the process of applying research results to clinical. How To Analyze Clinical Trial Data.
From www.mcdougallscientific.com
The Statistician’s view of a Clinical Trial How To Analyze Clinical Trial Data What is a clinical trial? This article reviews key portions of the process of applying research results to clinical practice. Assuming that a clinical trial will produce data. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. A clinical trial is any research study that prospectively assigns human participants or groups of humans to. How To Analyze Clinical Trial Data.
From ccrps.org
Fundamentals of Clinical Trials Phases of Clinical Trials CCRPS How To Analyze Clinical Trial Data A clinical trial is any research study that prospectively assigns human participants or groups of humans to one or. In publications of clinical trials, the first table in the paper usually summarizes the characteristics of the study sample, and readers should. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. Assuming that a clinical. How To Analyze Clinical Trial Data.
From pubrica.com
usingrprogrammingtoanalyzeclinicaltrialdata How To Analyze Clinical Trial Data Assuming that a clinical trial will produce data. In publications of clinical trials, the first table in the paper usually summarizes the characteristics of the study sample, and readers should. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. There are two types of data analyses of randomized clinical trials (rcts). This article reviews. How To Analyze Clinical Trial Data.
From pharpoint.com
The Journey of Clinical Trial Data Points From Patient to TLFs PharPoint How To Analyze Clinical Trial Data This article reviews key portions of the process of applying research results to clinical practice. Assuming that a clinical trial will produce data. The first step involves defining the study population. A clinical trial is any research study that prospectively assigns human participants or groups of humans to one or. Clinicians must be able to critically appraise clinical trials to. How To Analyze Clinical Trial Data.
From www.fiercebiotech.com
How to Make the Most of Your Clinical Trial Data—All of it Fierce Biotech How To Analyze Clinical Trial Data What is a clinical trial? Assuming that a clinical trial will produce data. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. A clinical trial is any research study that prospectively assigns human participants or groups of humans to one or. There are two types of data analyses of randomized clinical trials (rcts). Clinicians. How To Analyze Clinical Trial Data.
From statswork.com
What are different phases of clinical trials and how to design and How To Analyze Clinical Trial Data Clinicians must be able to critically appraise clinical trials to determine their internal validity. In publications of clinical trials, the first table in the paper usually summarizes the characteristics of the study sample, and readers should. What is a clinical trial? This article reviews key portions of the process of applying research results to clinical practice. A clinical trial is. How To Analyze Clinical Trial Data.
From clinnovo.blogspot.com
Clinnovo News Clinical Trial Data Analysis Using SAS Workflow How To Analyze Clinical Trial Data A clinical trial is any research study that prospectively assigns human participants or groups of humans to one or. The first step involves defining the study population. Assuming that a clinical trial will produce data. Clinicians must be able to critically appraise clinical trials to determine their internal validity. There are two types of data analyses of randomized clinical trials. How To Analyze Clinical Trial Data.
From www.slidemembers.com
Clinical Trial RoadmapTablesDiagram How To Analyze Clinical Trial Data This article reviews key portions of the process of applying research results to clinical practice. A clinical trial is any research study that prospectively assigns human participants or groups of humans to one or. Clinicians must be able to critically appraise clinical trials to determine their internal validity. The first step involves defining the study population. What is a clinical. How To Analyze Clinical Trial Data.
From www.clinicalleader.com
Data Interoperability The First Step To Leverage ML AI In Clinical Trials How To Analyze Clinical Trial Data There are two types of data analyses of randomized clinical trials (rcts). Assuming that a clinical trial will produce data. Trials should adhere to the consort statement. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. What is a clinical trial? In publications of clinical trials, the first table in the paper usually summarizes. How To Analyze Clinical Trial Data.