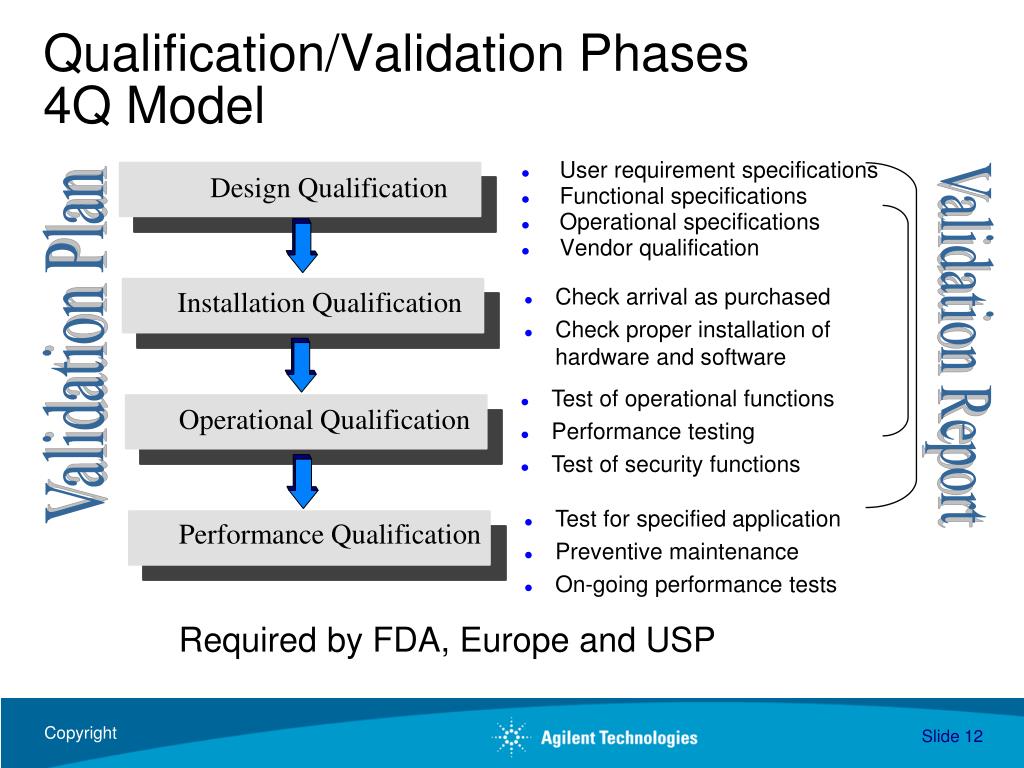
What Is Analytical Instrument Qualification . Discusses what a laboratory must do to ensure that qualified analytical instruments and validated computerized systems are set up and. Analytical instrument qualification forms the base for generating quality data. A large variety of analytical instruments, ranging from a simple apparatus. Analytical instruments should be qualified to demonstrate suitability for the intended use. The other components essential for generating quality data are. You may be unsure on what exactly to qualify or re. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in. The us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended.
from www.slideserve.com
Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in. A large variety of analytical instruments, ranging from a simple apparatus. The us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and. Discusses what a laboratory must do to ensure that qualified analytical instruments and validated computerized systems are set up and. Analytical instruments should be qualified to demonstrate suitability for the intended use. Analytical instrument qualification forms the base for generating quality data. You may be unsure on what exactly to qualify or re. The other components essential for generating quality data are.
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and
What Is Analytical Instrument Qualification You may be unsure on what exactly to qualify or re. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. A large variety of analytical instruments, ranging from a simple apparatus. The other components essential for generating quality data are. Analytical instrument qualification forms the base for generating quality data. You may be unsure on what exactly to qualify or re. Analytical instruments should be qualified to demonstrate suitability for the intended use. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in. Discusses what a laboratory must do to ensure that qualified analytical instruments and validated computerized systems are set up and. The us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and.
From www.youtube.com
New USP 1058 Analytical Instrument Qualification Regulations YouTube What Is Analytical Instrument Qualification Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Discusses what a laboratory must do to ensure that qualified analytical instruments and validated computerized systems are set up and. Analytical instruments should be qualified to demonstrate suitability for the intended use. You may be unsure on what exactly to qualify or. What Is Analytical Instrument Qualification.
From www.slideshare.net
Analytical instrument qualification PPT What Is Analytical Instrument Qualification Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. A large variety of analytical instruments, ranging from a simple apparatus. The us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in.. What Is Analytical Instrument Qualification.
From present5.com
AIQ Analytical Instrument Qualification AIQ Analytical What Is Analytical Instrument Qualification The us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and. You may be unsure on what exactly to qualify or re. Analytical instruments should be qualified to demonstrate suitability for the intended use. Analytical instrument qualification forms the base for generating quality data. A large variety of analytical instruments, ranging from a simple. What Is Analytical Instrument Qualification.
From www.slideshare.net
Analytical instrument qualification PPT What Is Analytical Instrument Qualification Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in. A large variety of analytical instruments, ranging from a simple apparatus. You may be unsure on what exactly to qualify or re. Analytical instruments should be qualified to demonstrate suitability for the intended use. Discusses what a laboratory must do to ensure that qualified analytical instruments. What Is Analytical Instrument Qualification.
From www.slideshare.net
Analytical instrument qualification PPT What Is Analytical Instrument Qualification The other components essential for generating quality data are. Analytical instrument qualification forms the base for generating quality data. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in. The us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and. You may be unsure on what exactly to qualify or. What Is Analytical Instrument Qualification.
From www.metrohm.com
Introduction to Analytical Instrument Qualification Part 1 Metrohm What Is Analytical Instrument Qualification The us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. A large variety of analytical instruments, ranging from a simple apparatus.. What Is Analytical Instrument Qualification.
From www.slideshare.net
Analytical instrument qualification What Is Analytical Instrument Qualification A large variety of analytical instruments, ranging from a simple apparatus. You may be unsure on what exactly to qualify or re. Analytical instrument qualification forms the base for generating quality data. The us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and. Analytical instrument qualification aiq is the collection of documented evidence that. What Is Analytical Instrument Qualification.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and What Is Analytical Instrument Qualification Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in. Analytical instrument qualification forms the base for generating quality data. The us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and. Analytical. What Is Analytical Instrument Qualification.
From www.slideshare.net
Analytical Instrument Qualification and System Validation What Is Analytical Instrument Qualification Analytical instrument qualification forms the base for generating quality data. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in. Discusses what a laboratory must do to ensure that qualified analytical instruments and validated computerized systems are set up and. The other components essential for generating quality data are. A large variety of analytical instruments, ranging. What Is Analytical Instrument Qualification.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and What Is Analytical Instrument Qualification You may be unsure on what exactly to qualify or re. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in. A large variety of analytical instruments, ranging from a simple apparatus. The us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and. Discusses what a laboratory must do to. What Is Analytical Instrument Qualification.
From docplayer.net
Analytical Instrument Qualification PDF Free Download What Is Analytical Instrument Qualification Analytical instruments should be qualified to demonstrate suitability for the intended use. Discusses what a laboratory must do to ensure that qualified analytical instruments and validated computerized systems are set up and. The other components essential for generating quality data are. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. You. What Is Analytical Instrument Qualification.
From www.scribd.com
Analytical Instrument Qualification (AIQ) in Pharmaceutical Industry What Is Analytical Instrument Qualification A large variety of analytical instruments, ranging from a simple apparatus. Discusses what a laboratory must do to ensure that qualified analytical instruments and validated computerized systems are set up and. You may be unsure on what exactly to qualify or re. The other components essential for generating quality data are. The us pharmacopeia (usp) general chapter on analytical instrument. What Is Analytical Instrument Qualification.
From www.scribd.com
ECA Analytical Instrument Qualification PDF Calibration Laboratories What Is Analytical Instrument Qualification You may be unsure on what exactly to qualify or re. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Discusses what a laboratory must do to ensure that qualified analytical instruments and validated computerized systems are set up and. The us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq). What Is Analytical Instrument Qualification.
From uspbpep.com
usp31nf26s1_c1058, General Chapters Analytical Instrument Qualification What Is Analytical Instrument Qualification A large variety of analytical instruments, ranging from a simple apparatus. Analytical instrument qualification forms the base for generating quality data. The other components essential for generating quality data are. Discusses what a laboratory must do to ensure that qualified analytical instruments and validated computerized systems are set up and. Analytical instrument qualification aiq is the collection of documented evidence. What Is Analytical Instrument Qualification.
From www.semanticscholar.org
AN OVERVIEW OF ANALYTICAL INSTRUMENT QUALIFICATION WITH REFERENCE OF What Is Analytical Instrument Qualification Analytical instrument qualification forms the base for generating quality data. A large variety of analytical instruments, ranging from a simple apparatus. Analytical instruments should be qualified to demonstrate suitability for the intended use. The other components essential for generating quality data are. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended.. What Is Analytical Instrument Qualification.
From www.youtube.com
Analytical instrument qualification presentation YouTube What Is Analytical Instrument Qualification Discusses what a laboratory must do to ensure that qualified analytical instruments and validated computerized systems are set up and. The other components essential for generating quality data are. A large variety of analytical instruments, ranging from a simple apparatus. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Us pharmacopeia. What Is Analytical Instrument Qualification.
From present5.com
AIQ Analytical Instrument Qualification AIQ Analytical What Is Analytical Instrument Qualification Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in. The us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and. A large variety of analytical instruments, ranging from a simple apparatus. Discusses what a laboratory must do to ensure that qualified analytical instruments and validated computerized systems are set. What Is Analytical Instrument Qualification.
From fwqrc.com
Guide on Analytical Instrument Qualification and System Validation What Is Analytical Instrument Qualification Analytical instrument qualification forms the base for generating quality data. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. A large variety of analytical instruments, ranging from a simple apparatus. The other components essential for generating quality data are. The us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was. What Is Analytical Instrument Qualification.
From www.learngxp.com
Equipment Qualification for Analytical Laboratory Instruments What Is Analytical Instrument Qualification Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. You may be unsure on what exactly to qualify or re. The us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and. The other components essential for generating quality data are. Us pharmacopeia (usp) general chapter. What Is Analytical Instrument Qualification.
From www.slideshare.net
Analytical instrument qualification PPT What Is Analytical Instrument Qualification Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in. The other components essential for generating quality data are. You may be unsure on what exactly to qualify or re. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Analytical instrument qualification forms the base for generating. What Is Analytical Instrument Qualification.
From www.researchgate.net
(PDF) AN OVERVIEW OF ANALYTICAL INSTRUMENT QUALIFICATION WITH REFERENCE What Is Analytical Instrument Qualification The other components essential for generating quality data are. Analytical instruments should be qualified to demonstrate suitability for the intended use. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in. Analytical instrument qualification forms the base for. What Is Analytical Instrument Qualification.
From www.metrohm.com
Introduction to Analytical Instrument Qualification Part 2 Metrohm What Is Analytical Instrument Qualification Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in. Analytical instrument qualification forms the base for generating quality data. You may be unsure on what exactly to qualify or re. The other components essential for generating quality data are. A large variety of analytical instruments, ranging from a simple apparatus. The us pharmacopeia (usp) general. What Is Analytical Instrument Qualification.
From www.semanticscholar.org
Analytical Instrument Qualification Standardization on the 4Q Model What Is Analytical Instrument Qualification You may be unsure on what exactly to qualify or re. Discusses what a laboratory must do to ensure that qualified analytical instruments and validated computerized systems are set up and. Analytical instruments should be qualified to demonstrate suitability for the intended use. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in. Analytical instrument qualification. What Is Analytical Instrument Qualification.
From www.industrialpharmacist.com
Analytical Instrument Qualification vs System Suitability Testing What Is Analytical Instrument Qualification The other components essential for generating quality data are. You may be unsure on what exactly to qualify or re. The us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and. Discusses what a laboratory must do to ensure that qualified analytical instruments and validated computerized systems are set up and. Analytical instruments should. What Is Analytical Instrument Qualification.
From www.slideshare.net
Analytical Instrument Qualification USP chapter 1058 revision What Is Analytical Instrument Qualification Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Analytical instruments should be qualified to demonstrate suitability for the intended use. Analytical instrument qualification forms the base for generating quality data. The other components essential for generating quality data are. Discusses what a laboratory must do to ensure that qualified analytical. What Is Analytical Instrument Qualification.
From www.slideshare.net
Qualification of analytical instrument of FTIR PPT What Is Analytical Instrument Qualification Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Analytical instrument qualification forms the base for generating quality data. The us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and. Analytical instruments should be qualified to demonstrate suitability for the intended use. A large variety. What Is Analytical Instrument Qualification.
From present5.com
AIQ Analytical Instrument Qualification AIQ Analytical What Is Analytical Instrument Qualification A large variety of analytical instruments, ranging from a simple apparatus. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in. You may be unsure on what exactly to qualify or re. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Analytical instrument qualification forms the base. What Is Analytical Instrument Qualification.
From www.slideshare.net
Qualification of analytical instrument of FTIR PPT What Is Analytical Instrument Qualification Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Discusses what a laboratory must do to ensure that qualified analytical instruments and validated computerized systems are set up and. A large variety of analytical instruments, ranging from a simple apparatus. The other components essential for generating quality data are. You may. What Is Analytical Instrument Qualification.
From www.scribd.com
Analytical Instrument Qualification USP 1058 LHEN Proposal PDF What Is Analytical Instrument Qualification Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in. Discusses what a laboratory must do to ensure that qualified analytical instruments and validated computerized systems are set up and. Analytical instrument qualification forms the base for generating quality data. You may be unsure on what exactly to qualify or re. A large variety of analytical. What Is Analytical Instrument Qualification.
From www.spectroscopyeurope.com
Instrument qualification a possible quality by designbased approach What Is Analytical Instrument Qualification The us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in. Discusses what a laboratory must do to ensure that qualified analytical instruments and validated computerized systems are set up and. Analytical instruments should be qualified to demonstrate suitability for. What Is Analytical Instrument Qualification.
From www.researchgate.net
(PDF) Analytical instrument qualification (AIQ) in pharmaceutical industry What Is Analytical Instrument Qualification Analytical instruments should be qualified to demonstrate suitability for the intended use. Analytical instrument qualification forms the base for generating quality data. You may be unsure on what exactly to qualify or re. The us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and. A large variety of analytical instruments, ranging from a simple. What Is Analytical Instrument Qualification.
From www.slideshare.net
Qualification of analytical instrument of FTIR PPT What Is Analytical Instrument Qualification You may be unsure on what exactly to qualify or re. Discusses what a laboratory must do to ensure that qualified analytical instruments and validated computerized systems are set up and. Analytical instrument qualification forms the base for generating quality data. Analytical instruments should be qualified to demonstrate suitability for the intended use. The other components essential for generating quality. What Is Analytical Instrument Qualification.
From www.slideshare.net
Analytical Instrument Qualification USP chapter 1058 revision PPT What Is Analytical Instrument Qualification Analytical instruments should be qualified to demonstrate suitability for the intended use. Discusses what a laboratory must do to ensure that qualified analytical instruments and validated computerized systems are set up and. The us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and. The other components essential for generating quality data are. Us pharmacopeia. What Is Analytical Instrument Qualification.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and What Is Analytical Instrument Qualification The other components essential for generating quality data are. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in. Analytical instrument qualification forms the base for generating quality data. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Analytical instruments should be qualified to demonstrate suitability for. What Is Analytical Instrument Qualification.
From www.slideshare.net
Analytical instrument qualification PPT What Is Analytical Instrument Qualification The other components essential for generating quality data are. You may be unsure on what exactly to qualify or re. The us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and. Analytical instruments should be qualified to demonstrate suitability for the intended use. Discusses what a laboratory must do to ensure that qualified analytical. What Is Analytical Instrument Qualification.