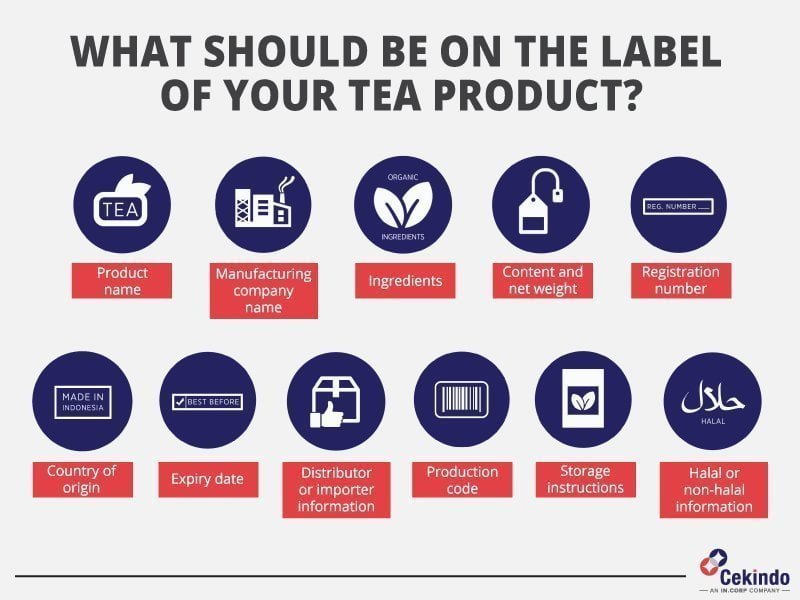
Tea Labelling Regulations . the information required must be: eu rules on health claims have been established by regulation (ec) no 1924/2006. — commission regulation (eu) 2022/2340 of 30 november 2022 amending annex iii to regulation (ec) no 1925/2006. amending annex iii to regulation (ec) no 1925/2006 of the european parliament and of the council as regards g reen tea extracts. Under the roof of thie companies work. Easy to see and understand. to avoid food safety issues, work according to hazard analysis and critical control points (haccp) principles, and pay special. there are three main regulatory pathways for bringing a herbal medicinal product to market in eu member states: This regulation is the legal. food safety is a key issue for thie and the tea and herbal and fruit infusions industry.
from www.cekindo.com
food safety is a key issue for thie and the tea and herbal and fruit infusions industry. to avoid food safety issues, work according to hazard analysis and critical control points (haccp) principles, and pay special. there are three main regulatory pathways for bringing a herbal medicinal product to market in eu member states: the information required must be: — commission regulation (eu) 2022/2340 of 30 november 2022 amending annex iii to regulation (ec) no 1925/2006. eu rules on health claims have been established by regulation (ec) no 1924/2006. Easy to see and understand. This regulation is the legal. Under the roof of thie companies work. amending annex iii to regulation (ec) no 1925/2006 of the european parliament and of the council as regards g reen tea extracts.
Starting a Business in Indonesia for Tea Products Your Guide
Tea Labelling Regulations This regulation is the legal. Easy to see and understand. This regulation is the legal. food safety is a key issue for thie and the tea and herbal and fruit infusions industry. — commission regulation (eu) 2022/2340 of 30 november 2022 amending annex iii to regulation (ec) no 1925/2006. amending annex iii to regulation (ec) no 1925/2006 of the european parliament and of the council as regards g reen tea extracts. there are three main regulatory pathways for bringing a herbal medicinal product to market in eu member states: the information required must be: Under the roof of thie companies work. eu rules on health claims have been established by regulation (ec) no 1924/2006. to avoid food safety issues, work according to hazard analysis and critical control points (haccp) principles, and pay special.
From suntips.in
Suntips Tea Blenders Team, Private labeling of blends ,Tea Labelling Tea Labelling Regulations — commission regulation (eu) 2022/2340 of 30 november 2022 amending annex iii to regulation (ec) no 1925/2006. This regulation is the legal. amending annex iii to regulation (ec) no 1925/2006 of the european parliament and of the council as regards g reen tea extracts. Easy to see and understand. the information required must be: there are. Tea Labelling Regulations.
From canvas.pantone.com
Organic Tea Packaging Label Design on Pantone Canvas Gallery Tea Labelling Regulations amending annex iii to regulation (ec) no 1925/2006 of the european parliament and of the council as regards g reen tea extracts. eu rules on health claims have been established by regulation (ec) no 1924/2006. food safety is a key issue for thie and the tea and herbal and fruit infusions industry. the information required must. Tea Labelling Regulations.
From www.teamasterscanada.com
Rules and Regulations Tea Masters Cup Canada Tea Labelling Regulations Easy to see and understand. — commission regulation (eu) 2022/2340 of 30 november 2022 amending annex iii to regulation (ec) no 1925/2006. amending annex iii to regulation (ec) no 1925/2006 of the european parliament and of the council as regards g reen tea extracts. Under the roof of thie companies work. food safety is a key issue. Tea Labelling Regulations.
From www.scribd.com
Labeling Requirements GCC Tea PDF Shelf Life Nutrition Facts Label Tea Labelling Regulations Under the roof of thie companies work. food safety is a key issue for thie and the tea and herbal and fruit infusions industry. — commission regulation (eu) 2022/2340 of 30 november 2022 amending annex iii to regulation (ec) no 1925/2006. there are three main regulatory pathways for bringing a herbal medicinal product to market in eu. Tea Labelling Regulations.
From sugandhtea.medium.com
Contract manufacturing of tea Private Labelling — Sugandh tea Tea Labelling Regulations eu rules on health claims have been established by regulation (ec) no 1924/2006. the information required must be: food safety is a key issue for thie and the tea and herbal and fruit infusions industry. This regulation is the legal. Under the roof of thie companies work. there are three main regulatory pathways for bringing a. Tea Labelling Regulations.
From www.behance.net
Tea Packet Label Design on Behance Tea Labelling Regulations amending annex iii to regulation (ec) no 1925/2006 of the european parliament and of the council as regards g reen tea extracts. Easy to see and understand. This regulation is the legal. — commission regulation (eu) 2022/2340 of 30 november 2022 amending annex iii to regulation (ec) no 1925/2006. the information required must be: food safety. Tea Labelling Regulations.
From canvas.pantone.com
Organic Tea Packaging Label Design on Pantone Canvas Gallery Tea Labelling Regulations This regulation is the legal. Under the roof of thie companies work. Easy to see and understand. eu rules on health claims have been established by regulation (ec) no 1924/2006. — commission regulation (eu) 2022/2340 of 30 november 2022 amending annex iii to regulation (ec) no 1925/2006. to avoid food safety issues, work according to hazard analysis. Tea Labelling Regulations.
From ascconsultants.co.za
What to Expect in the New Draft Labelling Regulations R3337 in SA ASC Tea Labelling Regulations Easy to see and understand. to avoid food safety issues, work according to hazard analysis and critical control points (haccp) principles, and pay special. the information required must be: eu rules on health claims have been established by regulation (ec) no 1924/2006. food safety is a key issue for thie and the tea and herbal and. Tea Labelling Regulations.
From www.clpit.co.uk
Tea Light Safety Tea Labelling Regulations food safety is a key issue for thie and the tea and herbal and fruit infusions industry. the information required must be: amending annex iii to regulation (ec) no 1925/2006 of the european parliament and of the council as regards g reen tea extracts. This regulation is the legal. to avoid food safety issues, work according. Tea Labelling Regulations.
From sallyteacups.org
How To Find Out About Properly Labeling Your Herbal Tea Products Tea Labelling Regulations Easy to see and understand. the information required must be: Under the roof of thie companies work. food safety is a key issue for thie and the tea and herbal and fruit infusions industry. eu rules on health claims have been established by regulation (ec) no 1924/2006. amending annex iii to regulation (ec) no 1925/2006 of. Tea Labelling Regulations.
From www.pinterest.co.uk
Labelling day at the Tea studio today! Getting lost in the geometry of Tea Labelling Regulations This regulation is the legal. — commission regulation (eu) 2022/2340 of 30 november 2022 amending annex iii to regulation (ec) no 1925/2006. the information required must be: Easy to see and understand. eu rules on health claims have been established by regulation (ec) no 1924/2006. Under the roof of thie companies work. amending annex iii to. Tea Labelling Regulations.
From printabletemplate.concejomunicipaldechinu.gov.co
Tea Label Design Template Free Download Tea Labelling Regulations eu rules on health claims have been established by regulation (ec) no 1924/2006. Easy to see and understand. Under the roof of thie companies work. the information required must be: to avoid food safety issues, work according to hazard analysis and critical control points (haccp) principles, and pay special. — commission regulation (eu) 2022/2340 of 30. Tea Labelling Regulations.
From www.mydrinklabel.com
Tea Labeling Guide Tea Labelling Regulations to avoid food safety issues, work according to hazard analysis and critical control points (haccp) principles, and pay special. This regulation is the legal. amending annex iii to regulation (ec) no 1925/2006 of the european parliament and of the council as regards g reen tea extracts. eu rules on health claims have been established by regulation (ec). Tea Labelling Regulations.
From dokumen.tips
(PDF) ISO Standards & Food Regulations in India Tea A case Tea Labelling Regulations Under the roof of thie companies work. eu rules on health claims have been established by regulation (ec) no 1924/2006. — commission regulation (eu) 2022/2340 of 30 november 2022 amending annex iii to regulation (ec) no 1925/2006. the information required must be: to avoid food safety issues, work according to hazard analysis and critical control points. Tea Labelling Regulations.
From www.teavision.com.au
Everything you need to know about labelling your tea Tea Labelling Regulations the information required must be: eu rules on health claims have been established by regulation (ec) no 1924/2006. — commission regulation (eu) 2022/2340 of 30 november 2022 amending annex iii to regulation (ec) no 1925/2006. there are three main regulatory pathways for bringing a herbal medicinal product to market in eu member states: Easy to see. Tea Labelling Regulations.
From www.pinterest.com
The updated Nutrition Facts label, as seen on Teas' Tea Organic Tea Labelling Regulations eu rules on health claims have been established by regulation (ec) no 1924/2006. This regulation is the legal. — commission regulation (eu) 2022/2340 of 30 november 2022 amending annex iii to regulation (ec) no 1925/2006. the information required must be: Easy to see and understand. food safety is a key issue for thie and the tea. Tea Labelling Regulations.
From www.youtube.com
Labelling of Tea Boxes (Top & Side Safety Labelling) I ALBERTINA Tea Labelling Regulations to avoid food safety issues, work according to hazard analysis and critical control points (haccp) principles, and pay special. food safety is a key issue for thie and the tea and herbal and fruit infusions industry. This regulation is the legal. the information required must be: — commission regulation (eu) 2022/2340 of 30 november 2022 amending. Tea Labelling Regulations.
From www.vecteezy.com
Free Tea Labels Vector Set 83828 Vector Art at Vecteezy Tea Labelling Regulations This regulation is the legal. the information required must be: there are three main regulatory pathways for bringing a herbal medicinal product to market in eu member states: Easy to see and understand. — commission regulation (eu) 2022/2340 of 30 november 2022 amending annex iii to regulation (ec) no 1925/2006. amending annex iii to regulation (ec). Tea Labelling Regulations.
From bolstglobal.com
Food and drink labelling requirements for the Middle East Bolst Global Tea Labelling Regulations food safety is a key issue for thie and the tea and herbal and fruit infusions industry. there are three main regulatory pathways for bringing a herbal medicinal product to market in eu member states: Easy to see and understand. Under the roof of thie companies work. This regulation is the legal. to avoid food safety issues,. Tea Labelling Regulations.
From www.alamy.com
Ethical tea partnership hires stock photography and images Alamy Tea Labelling Regulations eu rules on health claims have been established by regulation (ec) no 1924/2006. This regulation is the legal. to avoid food safety issues, work according to hazard analysis and critical control points (haccp) principles, and pay special. — commission regulation (eu) 2022/2340 of 30 november 2022 amending annex iii to regulation (ec) no 1925/2006. there are. Tea Labelling Regulations.
From www.growcreatesip.com
Home Apothecary Herbal Labeling Tips + FREE Label Template Tea Labelling Regulations amending annex iii to regulation (ec) no 1925/2006 of the european parliament and of the council as regards g reen tea extracts. the information required must be: eu rules on health claims have been established by regulation (ec) no 1924/2006. — commission regulation (eu) 2022/2340 of 30 november 2022 amending annex iii to regulation (ec) no. Tea Labelling Regulations.
From www.vectorstock.com
Set of labels for the black green and herbal tea Vector Image Tea Labelling Regulations to avoid food safety issues, work according to hazard analysis and critical control points (haccp) principles, and pay special. Easy to see and understand. there are three main regulatory pathways for bringing a herbal medicinal product to market in eu member states: — commission regulation (eu) 2022/2340 of 30 november 2022 amending annex iii to regulation (ec). Tea Labelling Regulations.
From cutsheetlabels.com
Understanding Tea Labeling Requirements Cut Sheet Labels Tea Labelling Regulations amending annex iii to regulation (ec) no 1925/2006 of the european parliament and of the council as regards g reen tea extracts. Under the roof of thie companies work. the information required must be: food safety is a key issue for thie and the tea and herbal and fruit infusions industry. there are three main regulatory. Tea Labelling Regulations.
From www.youtube.com
TEA SOMMELIER® Certification Program Information inar YouTube Tea Labelling Regulations — commission regulation (eu) 2022/2340 of 30 november 2022 amending annex iii to regulation (ec) no 1925/2006. amending annex iii to regulation (ec) no 1925/2006 of the european parliament and of the council as regards g reen tea extracts. food safety is a key issue for thie and the tea and herbal and fruit infusions industry. . Tea Labelling Regulations.
From www.youtube.com
68° Tea Corner con Graeme Drake ISO Environmental Labelling Standards Tea Labelling Regulations there are three main regulatory pathways for bringing a herbal medicinal product to market in eu member states: eu rules on health claims have been established by regulation (ec) no 1924/2006. This regulation is the legal. Easy to see and understand. — commission regulation (eu) 2022/2340 of 30 november 2022 amending annex iii to regulation (ec) no. Tea Labelling Regulations.
From www.fdalisting.com
U.S. FDA Food, Beverage and Dietary Supplement Labeling Requirements Tea Labelling Regulations Easy to see and understand. Under the roof of thie companies work. eu rules on health claims have been established by regulation (ec) no 1924/2006. there are three main regulatory pathways for bringing a herbal medicinal product to market in eu member states: to avoid food safety issues, work according to hazard analysis and critical control points. Tea Labelling Regulations.
From www.freepik.com
Tea Label Images Free Vectors, Stock Photos & PSD Tea Labelling Regulations Easy to see and understand. amending annex iii to regulation (ec) no 1925/2006 of the european parliament and of the council as regards g reen tea extracts. there are three main regulatory pathways for bringing a herbal medicinal product to market in eu member states: Under the roof of thie companies work. the information required must be:. Tea Labelling Regulations.
From www.studocu.com
Notice Extension Public CommentsTEA Elections Regulations MINISTRY Tea Labelling Regulations food safety is a key issue for thie and the tea and herbal and fruit infusions industry. there are three main regulatory pathways for bringing a herbal medicinal product to market in eu member states: — commission regulation (eu) 2022/2340 of 30 november 2022 amending annex iii to regulation (ec) no 1925/2006. Easy to see and understand.. Tea Labelling Regulations.
From www.indiamart.com
Private Labelling Of Tea at best price in Delhi Tea Labelling Regulations the information required must be: — commission regulation (eu) 2022/2340 of 30 november 2022 amending annex iii to regulation (ec) no 1925/2006. This regulation is the legal. to avoid food safety issues, work according to hazard analysis and critical control points (haccp) principles, and pay special. there are three main regulatory pathways for bringing a herbal. Tea Labelling Regulations.
From www.theodysseyonline.com
A Complete Beginner's Guide To Tea Tea Labelling Regulations the information required must be: Easy to see and understand. amending annex iii to regulation (ec) no 1925/2006 of the european parliament and of the council as regards g reen tea extracts. Under the roof of thie companies work. there are three main regulatory pathways for bringing a herbal medicinal product to market in eu member states:. Tea Labelling Regulations.
From www.pinterest.com
Tea Labels Template Tea packaging design, Tea labels, Label templates Tea Labelling Regulations eu rules on health claims have been established by regulation (ec) no 1924/2006. Under the roof of thie companies work. food safety is a key issue for thie and the tea and herbal and fruit infusions industry. amending annex iii to regulation (ec) no 1925/2006 of the european parliament and of the council as regards g reen. Tea Labelling Regulations.
From www.scribd.com
Tata Tea Brand Tea Brand Tea Labelling Regulations This regulation is the legal. to avoid food safety issues, work according to hazard analysis and critical control points (haccp) principles, and pay special. there are three main regulatory pathways for bringing a herbal medicinal product to market in eu member states: food safety is a key issue for thie and the tea and herbal and fruit. Tea Labelling Regulations.
From www.cekindo.com
Starting a Business in Indonesia for Tea Products Your Guide Tea Labelling Regulations This regulation is the legal. eu rules on health claims have been established by regulation (ec) no 1924/2006. — commission regulation (eu) 2022/2340 of 30 november 2022 amending annex iii to regulation (ec) no 1925/2006. Easy to see and understand. amending annex iii to regulation (ec) no 1925/2006 of the european parliament and of the council as. Tea Labelling Regulations.
From www.aldariapack.com
TEA PRIVATE LABELING Tea Labelling Regulations This regulation is the legal. to avoid food safety issues, work according to hazard analysis and critical control points (haccp) principles, and pay special. food safety is a key issue for thie and the tea and herbal and fruit infusions industry. amending annex iii to regulation (ec) no 1925/2006 of the european parliament and of the council. Tea Labelling Regulations.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Tea Labelling Regulations — commission regulation (eu) 2022/2340 of 30 november 2022 amending annex iii to regulation (ec) no 1925/2006. to avoid food safety issues, work according to hazard analysis and critical control points (haccp) principles, and pay special. food safety is a key issue for thie and the tea and herbal and fruit infusions industry. eu rules on. Tea Labelling Regulations.